Copley Scientific introduces new, upgraded breath simulators for inhaled product testing
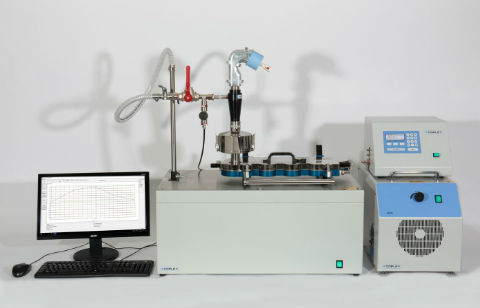
a test set-up optimised for improved IVIVCs, incorporating the BRS3100 and the adult Alberta Idealised Throat.
The upgraded BRS simulators make it even easier to apply specified inhalation profiles during OIP testing.
Copley Scientific has launched new, advanced breath simulators - the BRS 2100 and BRS 3100, for orally inhaled product (OIP) development and testing. In recent years, in response to regulatory and pharmacopoeial changes, and the drive to ensure closer correlation between in vivo and in vitro performance, scientists have increasingly incorporated breath simulators into their testing methods. Now, the upgraded BRS simulators from Copley Scientific make it even easier to apply specified inhalation profiles during OIP testing.
The BRS 2100 is ideally configured for testing nebulisers and metered dose inhalers (MDIs) with spacers/valved holding chambers (VHC) in accordance with US Pharmacopeia Chapter <1601> and Chapter <1602> respectively; the latter having only recently been released. The BRS 2100 has a maximum volume of 900 mL making it ideally suited to the low-volume, tidal breathing applications required by these chapters. The BRS 3100 offers greater volumes (500 mL up to 5000 mL) and the increased power (maximum flow rate of 240 L/min) needed for generating profiles typical of patients using MDIs and dry powder inhalers (DPIs) under forced inhalation conditions, helping to improve in vitro-in vivo correlations (IVIVCs).
As well as generating the ‘standard’ breathing profiles needed to measure delivered dose uniformity (DDU) according to pharmacopoeial requirements, namely: neonate, infant, child, and adult, for nebulisers and MDIs with spacers/VHCs, both the BRS 2100 and 3100 are designed to generate user-defined, patient-derived breathing profiles that more closely mimic real-world clinical situations. Importantly, the standard profiles can be used as a base and edited for development studies, adjusting the selected square, sinusoidal or triangular pattern, the tidal volume and the number, duration and timing of each breathing cycle, for example. For even more control, users can build and then save their own unique breathing profiles to simulate any desired pattern or breathing technique, as may have been recorded in clinic. Both breath simulators can be used for DDU applications or combined with a cascade impactor, using a mixing inlet, for aerodynamic particle size distribution (APSD) studies under more patient realistic conditions.
Compared with prior BRS 2000 and 3000 models, the BRS 2100 and BRS 3100 both feature an upgraded Quad-Core processor, increased RAM, improved Windows 10 operating system, and redesigned software, as part of the embedded, on-board computer. Significant gains in data processing speed and usability are the result. The instruments are also more flexible; for example, flow rates can be displayed in mL/s or L/min, and it is now easier to simulate uncoordinated product use, in the case of VHCs, by synchronising sampling with the exhalation portion of a breathing profile. In addition, the mechanics have been advanced – with improved motor, piston and cylinder performance enabling the more rigorous testing of high-resistance inhalers, and the creation of more powerful inhalation profiles. Additional peripherals and external data transfer are improved by the increased number of USB ports.
Mark Copley, Sales Director at Copley Scientific, comments: “In recent years, breath simulators have become a core piece of OIP testing equipment. This is partly because of the need to achieve better in vitro-in vivo relationships to enhance product development, and also in response to changing regulatory guidance, which has been updated for greater clinical relevance. Significant progress was made when the previous generation of breath simulators was introduced and now, with our upgraded series of instruments, we are very pleased to be putting state-of-the-art tools once again into the hands of our users.”
Related News
-
News Patients vs Pharma – who will the Inflation Reduction Act affect the most?
The Inflation Reduction Act brought in by the Biden administration in 2022 aims to give better and more equitable access to healthcare in the USA. However, pharma companies are now concerned about the other potential costs of such legislation. -
News CPHI Podcast Series: What does the changing US Pharma market mean for industry and patients alike?
In this week's episode of the CPHI Podcast Series Lucy Chard, Digital Editor for CPHI Online is joined by James Manser to discuss the political and market changes in the US pharma field. -
News CPHI Barcelona Annual Report illuminates industry trends for 2024
The CPHI Annual Survey comes into it’s 7th year to report on the predicted trends for 2024. Over 250 pharma executives were asked 35 questions, with their answers informing the industry landscape for the next year, spanning all major pharma marke... -
News Which 10 drugs are open to price negotiation with Medicare in the USA?
The Centres for Medicare & Medicaid Services, under the Biden administration in the USA, has released a list of the 10 drugs that will be open to price negotiations as part of the new legislation under the Inflation Reduction Act (IRA). -
News EU Medical Devices Regulation causes unintended disappearances of medical devices for children, doctors state
Doctor groups and associations have appealed to the EU to correct the EU Medical Devices Regulation law that may cause unintended shortages of essential drug and medical devices for children and rare disease patients. -
News 10 Major Drug Approvals So Far in 2023
Last year, 37 novel drugs were approved by the FDA, this was a high number for such a category, and covered many fields including oncology, demonstrating how promising further research is, and how it is only continuing to build. To date, there are alre... -
News Detecting Alzheimer's disease with a simple lateral flow test
A novel rapid diagnostic test for early-stage Alzheimer's disease has been developed using a biomarker binder from Aptamer Group along with technology from Neuro-Bio, the neurodegenerative disease experts. -
News CPHI Podcast Series: outsourcing and manufacturing trends
Listen to the CPHI Podcast Series this June to hear Gil Roth of the PBOA speak with Digital Editor Lucy Chard about the biggest trends and topics to watch in pharma outsourcing and manufacturing at the minute.
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance







