Copley Scientific releases upgraded SPU 2000 for reduced inhaler testing variability
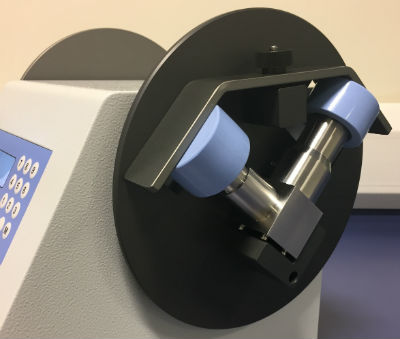
SPU 2000 FP Induction Port Attachment.
Company's modification makes the SPU 2000 a more cost effective laboratory tool.
Copley Scientific has upgraded its Sample Preparation Unit (SPU) Model 2000 to include a modified induction port fixture with a view to further broadening its appeal.
The upgraded SPU 2000 now accommodates a Fluticasone Propionate/Salmeterol Xinafoate (FP/SX) induction port, in addition to the standard Andersen Cascade Impactor (ACI) and Next Generation Impactor (NGI) induction ports, making it a universally applicable labour-saving device for rinsing the induction ports routinely used in cascade impactor testing. Copley Scientific produces equipment to improve in vitro testing of inhaled drug products and has upgraded the SPU 2000 to ensure accurate, reproducible and highly efficient testing across its cascade impactor range.
Conducting well-controlled, highly reproducible cascade impaction tests is critical for obtaining reliable data, for product development and quality control. Cascade impaction is used to measure the aerodynamic particle size distribution of inhaler generated aerosols and is a critically important procedure, but is widely recognised as being both time-consuming and manually intensive. Although not a technically complex task, recovering the active drug sample from the ACI and NGI induction ports, and NGI pre-separator (for which another fixture is available) is highly repetitive and susceptible to human error. Copley’s modified induction port fixture accommodates the new FP/SX induction port and addresses this industry challenge, by semi-automating the process, whilst reducing the potential for error associated with manual drug recovery and freeing up analysts to undertake other tasks.
“Fully automating cascade impactor testing can be challenging, because of its complexity and the need for complex validation and maintenance. We find that many of our customers regard semi-automation as a more practical and cost-effective option. Our latest modification to the SPU 2000 induction port fixture reflects the increasing use of the special FP/SX induction port. This modification enables semi-automated drug recovery from of a wider range of cascade impactor components, making the SPU 2000 an even more cost effective laboratory tool,” says Mark Copley, Sales Director at Copley Scientific.
Inhaled products containing FP and/or SX are currently among the most popular generic targets. This has resulted in the recent release of USP monographs, referencing drug specific cascade impactor components. Combined with the success of the SPU 2000 and increased customer demand, this is the key driver for the new upgrade. Optimisation of such labour-saving devices contributes to the cost-efficient implementation of vital testing techniques and enhances quality of results.
Related News
-
News Patients vs Pharma – who will the Inflation Reduction Act affect the most?
The Inflation Reduction Act brought in by the Biden administration in 2022 aims to give better and more equitable access to healthcare in the USA. However, pharma companies are now concerned about the other potential costs of such legislation. -
News CPHI Podcast Series: What does the changing US Pharma market mean for industry and patients alike?
In this week's episode of the CPHI Podcast Series Lucy Chard, Digital Editor for CPHI Online is joined by James Manser to discuss the political and market changes in the US pharma field. -
News CPHI Barcelona Annual Report illuminates industry trends for 2024
The CPHI Annual Survey comes into it’s 7th year to report on the predicted trends for 2024. Over 250 pharma executives were asked 35 questions, with their answers informing the industry landscape for the next year, spanning all major pharma marke... -
News Which 10 drugs are open to price negotiation with Medicare in the USA?
The Centres for Medicare & Medicaid Services, under the Biden administration in the USA, has released a list of the 10 drugs that will be open to price negotiations as part of the new legislation under the Inflation Reduction Act (IRA). -
News EU Medical Devices Regulation causes unintended disappearances of medical devices for children, doctors state
Doctor groups and associations have appealed to the EU to correct the EU Medical Devices Regulation law that may cause unintended shortages of essential drug and medical devices for children and rare disease patients. -
News 10 Major Drug Approvals So Far in 2023
Last year, 37 novel drugs were approved by the FDA, this was a high number for such a category, and covered many fields including oncology, demonstrating how promising further research is, and how it is only continuing to build. To date, there are alre... -
News Detecting Alzheimer's disease with a simple lateral flow test
A novel rapid diagnostic test for early-stage Alzheimer's disease has been developed using a biomarker binder from Aptamer Group along with technology from Neuro-Bio, the neurodegenerative disease experts. -
News CPHI Podcast Series: outsourcing and manufacturing trends
Listen to the CPHI Podcast Series this June to hear Gil Roth of the PBOA speak with Digital Editor Lucy Chard about the biggest trends and topics to watch in pharma outsourcing and manufacturing at the minute.
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance







