CPL SACHSE
About CPL SACHSE
Certifications
Categories

-
DE
-
2023On CPHI since
-
2Certificates
-
25 - 49Employees
Company types
Primary activities
Products from CPL SACHSE (3)
-
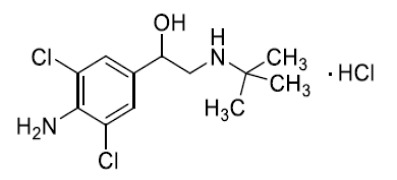
Product Clenbuterol hydrochloride
Our production follows cGMP and is in accordance with the EU-GMP-Guideline Part II (ICH Q7). We are regularly inspected by the responsible local authority which issues us with drug substance-specific GMP certificates. We are currently manu... -
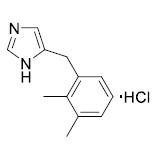
Product Detomidine hydrochloride
Our production follows cGMP and is in accordance with the EU-GMP-Guideline Part II (ICH Q7). We are regularly inspected by the responsible local authority which issues us with drug substance-specific GMP certificates. We are currently manu... -
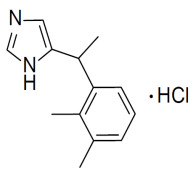
Product Medetomidine hydrochloride
Our production follows cGMP and is in accordance with the EU-GMP-Guideline Part II (ICH Q7). We are regularly inspected by the responsible local authority which issues us with drug substance-specific GMP certificates. We are currently manu...
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance