Daewoong Pharmaceutical Co.,Ltd
About Daewoong Pharmaceutical Co.,Ltd
Categories

-
KR
-
2015On CPHI since
-
1000 - 4999Employees
Company types
Primary activities
Meet us at
CPHI Milan 2024
Fiera Milano, Italy
08 Oct 2024 - 10 Oct 2024
Products from Daewoong Pharmaceutical Co.,Ltd (5)
-
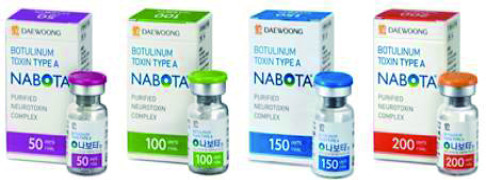
Product NABOTA®
Botulinum toxin type A
Indication: Glabella Lines (Approved in KR, US, CA, EU), Post Stroke Upper Limb Spasticity, Crow's feet, Blepharospasm, Benign Masseteric Hypertrophy (Approved in KR)
- Nabota is the only 900kDa Neurotoxin approved in the US and EU since Botox.- It has demonstra... -
Product CREZET
1) Indication : Treatment of primary hypercholesterolemia or to decrease elevated fat level in blood (mixed hyperlipidemia) in adult patients
2) Composition : Ezetimibe, Rosuvastain calcium (strength: 10/20mg, 10/10mg,10/5mg)
3) Dosage & Administration : Once a day with or without m... -
Product ENVLO®
Envlo (Enavogliflozin) is a best-in-class SGLT2 inhibitor for T2DM.
Highlights of Envlo are:
* Launched in Korea from May 2023
* Approved by Korea MFDS in 2022 for T2DM
* T2DM Phase III was completed in Korea as a monotherapy and in combinations with other anti-diabetics (du... -
Product Eposis
2000, 3000, 4000, 5000, 6000, 8000, 10000IU / Pre-filled syringe Inj.
rhEPO (recombinant human erythropoietin)
Indication:
Anemia in chronic renal failure
- EposisTM demonstrated significant improvement and safety for patients with anemia in Chronic Renal Failure (CRF).
... -
Product FEXUCLUE®
Fexuclue (Fexuprazan) is the best-in-class novel anti acid secretion agent with rapid onset time and potent acid suppressive effect, addressing the growing unmet needs of PPIs
Highlights of Fexuclue are: * Launched in Korea (2022) and Philippines (2023)
* Approved in Chile and Ecuador qq...
Daewoong Pharmaceutical Co.,Ltd Resources (1)
-
Video DAEWOONG PR VIDEO (EN)
We continue to take the initiative by making bold challenges and investments in R&D to leap forward as a global healthcare group. Following the 2022 launch of Fexuclue, a drug treating gastroesophageal reflux disease, the company released Envlo, a Type 2 diabetes treatment. By bolstering its R&D pipeline, Daewoong Pharmaceutical is developing first-in-class and best-in-class drugs.
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance