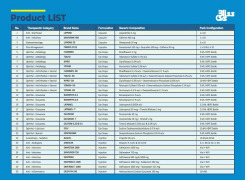
CPHI Online is the largest global marketplace in the pharma ingredients industry
-
Products551,744
-
Companies7,781
-
Articles11,636
-
Events8
-
Webinars342
Finished Dosage Forms
Finished Dosage Forms Companies (478)
Finished Dosage Forms News
-
News Providing solutions for special populations – CPHI North America Interview
In Philadelphia in May on site at CPHI North America we were able to meet with some of our speakers regarding their presentations. In the following interview Srinivasan Shanmugam, Executive Director, Pharmaceutical Sciences, Business Sup...10 Jun 2024 -
News Breaking Barriers: Innovations in Oral Solid Dose Form Bioavailability
The effectiveness of a medication often hinges on its bioavailability – the rate and extent at which the active ingredient is absorbed into the bloodstream. When it comes to oral solid dose forms, such as tablets and capsules, the challenge lies ...28 Aug 2023 -
News Rebuilding the United States' pharma infrastructure – CPHI North America 2023 preview
As we prepare for CPHI North America from April 25–27 in Philadelphia, USA, CPHI Online caught up with some of the track sponsors to discuss how the show connects vital players within the North American pharmaceutical landscape. ...18 Apr 2023 -
News Pharma Supply Chain People Moves
The latest appointments, promotions, and structural changes across the pharmaceutical supply chain.17 Apr 2023 -
Sponsored Content How healthcare trends inform dosage forms
Capsules encompass one of the most popular solid oral dosage forms for pharmaceutical products, with the global empty capsule market predicted to rise to USD $3.7 billion by 2026. The growth in the capsule market can be partly attributed to the many op...28 Feb 2023 -
News Ten23 health expands newly acquired sterile drug product manufacturing site
The expansion will provide additional cold storage and visual inspection capacity, as well as cleanrooms for device assembly and secondary packaging28 Mar 2022 -
News HCmed to expand inhalation treatment business with new CDMO offering
The Taiwan-based nebuliser specialist has signed an MoU with Formosa Laboratories, and Formosa Pharmaceuticals to form a biopharma one-stop-shop7 Mar 2022 -
News Recipharm streamlines operations by selling oral solid dosage manufacturing site
Astrea Pharma will be the new owner of the facility at Fontaine-lès-Dijon10 Jan 2022 -
News ANI Pharma enhances generics and CDMO business through Novitium acquisition
The transaction expands ANI’s R&D pipeline and adds nine new customers to its growing CDMO business24 Nov 2021 -
News Meiji Seika to expand CDMO business with new manufacturing facility in India
The $20.1 million investment will enable the company to manufacture pharma products for the Adcock Ingram Group and for other clients10 Nov 2021 -
News CPHI Worldwide is back, and this time it’s hybrid
A new in-person and online edition of CPHI Worldwide, transforming your event experience.1 Nov 2021 -
News Next stop, Milan - Welcome to Italy's life sciences hub
Milan is home to an internationally competitive pharmaceutical, biotechnology and functional foods hub, with strong growth prospects and a commitment to innovation across its entire supply chain. Italy is also home to one of the most advanced and ...21 Oct 2021 -
News MannKind signs sale-leaseback agreement for inhaled insulin manufacturing site
The transaction will generate $102.25 million to support the company's product pipeline and scaling up commercial activities for Afrezza1 Oct 2021 -
News Lonza to establish multi-product fill and finish line in China
The new production line at Guangzhou site will supply global and domestic companies with clinical and commercial batches23 Aug 2021 -
News Discover the CPHI North America Learning Labs: Part One
Explore the series of Learning Labs at CPHI North America across several product innovation categories in which thought leaders at our exhibitors showcase their extensive expertise in all areas of the pharma supply chain, offering industry insight...30 Jul 2021 -
News Resyca joint venture ready to push the boundaries of softmist technology
Joint venture between Recipharm and Medspray could provide significant advantages for patients and the environment, companies say1 Feb 2021 -
News CPHI Online Report - Pharma Market Trends 2021
Twelve pharma market trends we anticipate across 2021 for the drug development, manufacturing and outsourcing sectors.25 Nov 2020 -
News Recipharm signs up for aseptic fill-finish manufacturing of Moderna COVID-19 vaccine
The CDMO is already making certain investments to enable technology transfer and scale-up to commence imminently.24 Nov 2020 -
News Recipharm and Medspray establish joint venture to exploit novel softmist technology
Resyca BV will provide a "unique offering" to the market — environmentally friendly, softmist spray nozzle technology for pharmaceutical applications.17 Nov 2020
Finished Dosage Forms Products (1000+)
-
Product Sildenafil Citrate Oral Jelly - Sextreme XL 120 mg
Sextreme Oral Jelly 120mg is having the drug ingredient called Sildenafil Citrate and is available in a pack of 7 sachets a Box with different flavours. The product is available in Jelly form for easy intake. Contact us for more Information.
-
Product Tadalafil chewable tablets 20mg-Vikalis
Vikalis 20mg having the drug ingredient called Tadalafil 20mg is available in the tablet form and packed in a strip of 10 tabs. The product is also available in the strength of 5mg, 10mg, 20mg, 40mg and 60mg. Contact us for more Information.
-
Product Eslotin (Tablets)
Desloratadine, 2.5 or 5 mg.
Available in 10, 20, or 30 Film-Coated Tablets.
-
Product Besone 50 (Beclomethasone Dipropionate)
Midas Care offers a wide range of pMDI formulations which includes Beclomethasone Dipropionate 50mcg (Besone 50). It is an anti-asthmatic that works directly in the lungs to make breathing easier by reducing the irritation and swelling of the airways.Contact us for more information.
-
Product Salbesone (Beclometasone Dipropionate + Salbutamol Sulphate)
Midas Care offers a wide range of pMDI formulations which include Salbutamol 100mcg + Beclomethasone Dipropionate 50mcg (Salbesone). It is a synergistic combination which may be used effectively in prophylaxis of early & late asthmatic reactions. Contact us for more information.
-
Product Bacitracin vials
Bacitracin is comprised of a polypeptide complex and bacitracin A is the major component in this complex active against a variety of Gram-positive bacteria and a few Gram-negative bacteria, however only staphylococcal infections qualify for the systemic therapy.
Indi...
-
Product Colistimethate sodium lyophilized vials
Colistimethate sodium is a prodrug of colistin, which is a mixture of polypeptide antibiotics active against Gram-negative bacteria, including Enterobacter aerogenes, Escherichia coli, Klebsiella pneumoniae and especially Pseudomonas aeruginosa.
Indication:&nbs...
-
Product INTELECTA (L-Carnitine)
INDICATIONS: Levocarnitine deficiency.
INJECTABLE AMPOULES:
1g L-Carnitine/5ml (BT x 6 ampoules)
2g L-Carnitine/5ml (BT x 6 ampoules)
ORAL SOLUTION SINGLE DOSE:
1g L-Carnitine/10ml monodosimetric vial (BT x 10 divided dose vials)
2g L-Carnitine/10ml monodosimetric vial...
-
Product HEMAFER (Ferric Hydroxide Polymaltose Complex)
INDICATIONS: Iron deficiency of any cause and iron deficiencies without anaemia.
CHEWABLE TABLETS: 100mg Iron(III)/tablet (BT x 30 chew. tabl.)(BT x 50 chew. tabl.)(BT x 100 chew. tall.)ORAL SOLUTION SINGLE DOSE: 100mg Iron(III)/5ml (BT x 10 vials)...
-
Product Softgels
Softgel Capsules
EnterlCare® Enteric Softgels
Twist-Off Softgels
LiquilSoft™ Chewable Liquid-Filled Softgels
Versatrol™ Controlled-Release Softgels
Solvatrol™ Enhanced Solubility Softgels
Soflet® Gelcaps
Chewels® Chewable Gels
EcoCaps™ Non-Animal Softgels
-
Product Broncositol® oral supplement
The only myo-inositol-based dietary supplement specifically designed to manage respiratory diseases
Supports the maintenance of the physiological state of the respiratory system by promoting the health of mucous membranes and the production of lung surfactants
The inclu...
-
Product Sterile drug product CDMO services
Thermo Fisher Scientific's flexible aseptic manufacturing and sterile fill finish solutions for your molecule’s unique needs and challenges will enable success in early development, late-phase, and commercial manufacturing.
Thermo Fisher offers extensive sterile product development and commercial ...
-
Product Inofolic ® & Inofolic ® HP
A breakthrough in the managementof PCOS and female infertility
• Pharmaceutical form: Sachets | Soft-gel caps • Clinical trials: Efficacy of the product line is supported by 60+ international clinical trials in more than 5000 patients. Many published studies demonstrate efficacy in imp...
-
Product VORENT (voriconazole) 200 MG FILM COATED TABLET
VORICONAZOLE 200 MG FILM COATED TABLET (30 TABS)
VORENT is a broad-spectrum antifungal agent belonging to the triazole group, indicated for the treatment of the following fungal infections:
• Treatment of invasive aspergillosis, • Treatment of candidemia in non-neutropenic patients...
-
Product Gelee Royale Cardio
Key Ingredients of Gelee Royale Cardio:
• Royal Jelly: The highest content of royal jelly (2000 mg) and HDA (28 mg) so far • Coenzyme Q10: Known for its heart-protective and cardiovascular functions • Vitamins B1, B6, D3, K2, and E: Essential for various aspects of heart and overall health. ...
-
Product Liraglutide injection
for Diabetes and weight loss indication, China marketed drug. CTD form dossier
-
Product We take your products from idea to market success
Hänseler Swiss Pharma is a dedicated partner known for its unwavering commitment to the highest quality standards and innovative strategies. We bring our enthusiasm and Swiss precision to support our partners across the entire value chain, providing custom-tailored solutions for your success in the pha...
-
Product Passionis
Passionis is a unique product specially for women, which have been related to sexual appetite boost properties, containing Liboost, Maca, Vitamin B6 and Zinc.
-
Product Cogiton
Cogiton is a patented food supplement based on a specific and harmonized combination of Ginkgo Biloba, B vitamins, carnosine, coenzyme Q10, L-cysteine, beta-carotene, vitamin E, vitamin C and selenium.
Cogiton is a drinkable food supplement available in PET vials with a dosing cap, easy to us...
-
Product FREE NOSE® ANTI ALLERGY
Prevents and relieves mucosa against allergens and symptoms of allergic rhinitis (against pollen, mites and/or pet hair) while maintaining the mucosa fresh and moisturized.
Contains aloe vera and xanthan gum, as well as eucalyptus, niaouli, lemon and mint essential oils.
20 ml nas...
-
Product Xerthra™ kits
Xerthra™ kits – procedure packs for isolation and separation of platelet rich plasma (PRP) or injectible fibrine (iPRF) from patient’s peripheral blood.
Xerthra™ PRP kit provides a fraction of autologus plasma with increased number of leukocytes which synthesize cytokines and higher amounts ...
-
Product Scientific Support
Selectchemie AG provides a wide range of services including scientific support. Our experienced professionals are at your service at locations in 18 countries to deliver tailor-made solutions according to your needs. Contact us for more information.
-
Product CordenPharma Oral Formulation Tablets & Capsule Drug Products
CordenPharma’s service offerings include a wide range of products, formulations and services supporting new chemical entities, re-formulations and generics:
• Powder & Granule-filled Capsules • Powder Granules & Pellets • Immediate Release Tablets • Controlled Release Tablets • Mini-tablets...
-
Product FINISHED FORMULATION , PHARMACEUTICALS , CONTRACT MANUFACTURING
FINISHED PHARMA FORMULATION : TABLETS, CAPSULES, SEMI SOLIDS ARE EU GMP APPROVED.WE ALSO HAVE DRY SYRUP, LIQUIDS, STERILE ( SVP, LVP , EYE / EAR) DROPS WHO cGMP . FORMULATON OF VARRIED THERAPEUTIC SEGMENTS .
WE CONTRACT MANUFACTURE OTC ( orals, ointments, ...
-
Product LOCASALENE 0.2 MG/G CREAM
It is used in subacute and chronic inflammatory skin diseases that respond to corticosteroids, especially with hyperkeratosis.
-
Product Irbesartan + Hydrochlorothiazide, 150mg + 12,5 mg tablets
Treatment of essential hypertension.
This fixed-dose combination is indicated in adult patients whose blood pressure can not be adequately controlled only by irbesartan or hydrochlorothiazide. ATC Code C09D A04.
10 tablets in the blister, 30 tablets in the pack
...
-
Product Abacavir Sulfate
Hetero Drugs Limited offers a wide range of finished dosage which includes abacavir sulfate. It is a type of anti-inflammatory/ muscle relaxants and paediatric formulations. Form: tab/oral sol. Contact us for more information.
-
Product Blow-Fill-Seal Technology (BFS) Single-dose vials CDMO
Salvat specializes in developing and manufacturing sterile liquid pharmaceutical products in single-dose vials using Blow-Fill-Seal (BFS) technology.
Advantages of using Blow-Fill-Seal technology in your products:
• • Innovative format, providing differentiation for your produ...
-
Product Retico® - Eye Health Support
Retico® line is a comprehensive solution designed to support eye health and prevent eye diseases. Developed through rigorous scientific research and clinical trials, Retico® combines standardized extracts from berry fruits known for their potent antioxidant and anti-inflammatory properties. The Retico...
-
Product GYNELLA Flora
Innovative Medical device based on non-viable tyndallized lactic strains (Lactobacillus acidophilus, Lactobacillus reuteri, Lactobacillus casei) to support natural protective mechanisms of vaginal microflora in time when the defence is reduced due to external factors. The non-viable strains are aligned wit...
-
Product ARTILANE CLASSIC - HELPS TO PREVENTS JOINT WEAR AND IMPROVES JOINT FLEXIBILITY AND MOVEMENT
COMPOSITION: Enzymatic hydrolyzed collagen of high purity, Hyaluronic acid, Antioxidants (Quercetine, Lycopene, Vitamin C, Coenzyme Q10), Magnesium and Zinc.
INDICATION: dietary supplement for osteoarthritis treatment based on Collagen, Hyaluronic acid and Antioxidant that improves functional ...
-
Product UniLayer - 2mg Loperamide
This product is suitable for children as a delivery system.
Must be taken with water or beverage of choice.
We are looking to partners on a CDMO bases.

-
Product Nexicure - esomeprazole 40 mg and 20 mg MUPS tab
Nexicure 40 mg and 20mg MUPS tabPack: 20 tab
Category: proton pump inhibitors (PPIs)The first and only PPI - Esmoperazole 40 mg and 20mg tab generic with MUPS technology
Indication: It is used to treat gastroesophageal reflux disease GERD, peptic ulcer disease, and&nbs...
-
Product Melatonin 1mg/ml oral solution
Sleep onset insomnia in children and adolescents (6-17 years of age) with attention deficit hyperactivity disorder (ADHD) where other healthy sleeping routines have not worked well enough.
Short-term treatment of jet lag in adults. -
Product SOPA-K®
Iasis Pharma S.A. provides wide range of products which includes Sopa-K® (Active ingredient; potassium gluconate). It prevents and treats hypokalemia. Contact us for more information.

-
Product Diclofenac / Omeprazole, HGC, modified release
Pharmaceutical Licensing Product
Chemical for symptomatic treatment of RA, osteoarthritis, ankylosing spondylitis
Aenova offers a broad range of pharmaceutical license products under the following license model:
• Aenova provides and updates dossiers and scientific materia...
-
Product MILEDIX®
Miledix ® is a food supplement formulated for well-being during menstrual cycle.
Miledix ® is indicated for the women effected of premenstrual syndrome and dysmenorrhea.
The major clinical symptoms are grief, irritability, depression, abdominal cramps, mood changes, low so...
-
Product Vitamin Premix Powder
Customized premix solution in powder
We bring you custom nutrient premix blends sourced from high quality ingredients,including vitamins,minerals,amino acids,nucletide,nutraceutical,proteins,sweeteners,prebiotic,fibers,herbs, and more.
-comp286402.jpg)
-
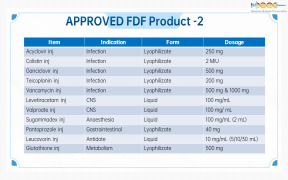
Product Genovior Products - FDF Drugs List 2
Acyclovir inj
Colistin inj
Ganciclovir inj
Teicoplanin inj
Vancomycin inj
Levetiracetam inj
Valproate inj
...
-
Product Salbutamol Inhalation Aerosol
It is used to prevent and treat respiratory diseases associated with bronchospasm (wheezing) such as bronchial asthma or asthmatic bronchitis.

-
Product Betahistine 12.5 mg/ml Oral Drops
Glass bottle containing 100 ml oral solution with disposal pump.
-
Product 2024- TPA Dosage Forms list
Connecting Taiwan’s pharmaceutical manufacturers with the global marketplace through various business models such as In-Out Licensing, Tech-Transfer, Contract Manufacturing Organization (CMO), and Contract Development and Manufacturing Organization (CDMO). We collaborate closely not only with local manufac...
-
Product Your UTI Free Orosticks
Natural solution for UTI prevention or acute use with an outstanding track record of market response. Supported by 2 clinical studies on the finished product. Launches in various regions are showing exceptional prescription rates and sales due to the product’s efficacy. Based on an exclusive, branded ...
-
Product OPHTHA HYAL Line - THE EYE, WINDOW TO THE SOUL, WINDOW TO THE WORLD
SafloHyal - in case of OCULAR INFLAMMATION
Key Ingredients: Enoxolone glucuronide 2.5 %, Hyaluronic Acid Sodium Salt 0.10%, PEG/PPG (EG56) 1.5%.
OftaxHyal - in case of CORNEAL INJURIES
Key Ingredients: Hyaluronic Acid, Glyceryl...
-
Product FDF - onoclogy , Diabetic , Anti malaria ,
general catagury API we offer API Acyclovir
Deferaserox
Artimether
Lumfentrine
• www.globelapharma.com
-
Product Innovative Food Supplements
GAP offer its own brand for Food Supplements for Distribution or Pvt LabelOur formulas developed are ALL based on EFSA claims and cover many therapeutical categories: (ex. Mental Health, Sleep Disorders, Boosting Imune System etc)
For full range please refer of the formulas we offer please g...
-
Product Redulen® Glicemia
Redulen® Glycemia is an innovative food supplement for blood glucose control based on:
• Lemotrin™ a proprietary synergistic complex of flavonoids, made of Mediterranean lemon and orange extracts • Chromium contributing to the maintenance of normal blood glucose levels* Redulen® ...
-
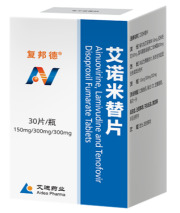
Product Ainuomiti Tablets
1. The first-ever Chinese original anti-HIV/AIDS fixed-dose combination (Ainuovirine, Lamivudine and Tenofovir Disoproxil Fumarate Tablets)
2. One tablet a day, easy to take
3. In line with the international mainstream Antiretroviral Therapy (ART) Regimens
4. Preferred regimen...
-
Product Cross-Linked Intra Articular Gel | VISCOSUPPLEMENT | Modified Hyaluronic Acid 2-5 mL | 10-120 mg
SEMICAL® Gel-B Cross Sodium Hyaluronate for Intra-Articular Injection
Mimics Synovial Fluid!
Advanced Thixotropic Process Makes it Unique!
SEMICAL® intra-articular Gel-B Cross is a highly purified bio-fermented sodium hyaluronate based viscoelastic solution ...
-
Product Registration Dossiers / Finished Dosage Forms
Clopidogrel TabletsDexketoprofen Tablets
Dimenhydrinate Tablets
Fexofenadine Tablets
Lisinopril + HCTZ Tablets
Loratadine Tablets
Memantine Tablets
Minoxidil Cutaneous Spray
Perindopril Tablets
Perindopril + Indapamide Tablets
Perindopril + Amlodipine Tablets
Rivaroxaban Table... -
Product Mirabegron (ER) 25 mg/50 mg and Solifenacin 5 mg/5 mg Tablet
Fixed dose combination (FDC) of Mirabegron (Extended release) and Solifenacin tablet can be used for the treatment of overactive bladder and to control the frequent urination. These drugs act together by their distinct mechanism of action that provide clear improvement and reduce side effects of m...
-
Product medEctoin Allergy Eye Drops
Medical device for treatment and prevention of allergic conjunctivis symtoms.
• Quick symptoms reduction like itching, tearing and irritation within 30 seconds • Alleviates allergic symptoms (red, itchy and watery eyes) • Reduces allergen induced inflammations of the conjunctiv...
-
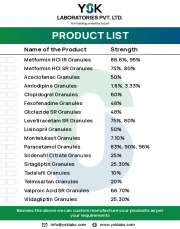
Product Directly Compressible Granules
1. Aceclofenac Granules 50%2. Clopidogrel Granules 60%
3. Fexofenadine Granules 48%
4. Gliclazide Granules SR 48%
5. Levitiracetam Granules SR 75%, 80%
6. Lisinopril Granules 50%
7. Metformin HCL Granules IR 86.66%, 93%
8. Metformin HCL Granules SR 75%, 80%
9. Montelukast Granules 7.1%...
-
Product Amnion Flush Solution®
Amnion Flush Solution® for continuous amnioinfusion is the first certified treatment option that permanently restores the physiological foetal environment after the preterm premature rupture of membranes (PPROM).Continuous amnioinfusion regenerates the natural amniotic fluid depot via a subcutaneously impl...
-
Product Sinomarin® Plus Algae -seawater nasal sprays with sea algae extracts
Sinomarin Plus Algae is an innovative product line combining a 2.3% hypertonic seawater solution with a unique ingredient complex based on sea algae extracts -Algomer Complex.
Sinomarin Plus Algae products decongest the nose naturally while providing additional strength and protection to th...
-
Product Gyno-Balance - pH regulator
Non – hormonal vaginal gel specially formulated to restore physiological vaginal pH and balance its bacterial flora.
Indicated in case of bacterial vaginosis, vaginitis and vaginal mycosis. It prevents relapses, especially following antibiotics treatments. The vaginal pH is an important factor f...
-
Product ABINCOL
ABINCOL is a food supplement whose composition is based on probiotic lactic ferments useful for restoring and maintaining the balance of the intestinal bacterial flora.
Pack and mode of use:
The package contains 20 buccal sticks and the recommended dosage is 1 stick per day, preferably...
-
Product Aripiprazole Oral Suspension
Rubicon provides a wide range of central nervous system products which includes aripiprazole ODT. It belongs to Abilify, Otsuka brand. Contact us for more information.
-
Product Effervescent Tablets & Sachets - Acetylcysteine, Multivitamins with minerals, Vitamin range
Wide range of Products with different Flavors - Berry, Orange & Cherry.
-
Product Kleen Enema / Laxative Enema / Monobasic Sodium / Dibasic Sodium Phosphate
Rectal Solution 135Ml
-
Product Kopaq (Iohexol), Concentrated Solution for Injection - Vial, 300 mgI/ml (50 ml), 300 mgI/ml (100 ml), 350 mgl/ml (50 ml), 350 mgl/ml (100 ml), 350 mgl/ml (200 ml)
Kopaq (Iohexol), Concentrated Solution for Injection - Vial, 300 mgI/ml (50 ml), 300 mgI/ml (100 ml), 350 mgl/ml (50 ml), 350 mgl/ml (100 ml), 350 mgl/ml (200 ml) is a iodinated, nonionic, low osmolar contrast agent.The product is used in arthrography, endoscopic retrograde pancreatography (ERP), endoscopi...
-
Product Glutaredox
Food supplement based on reduced Glutathione, Vitamin C,L-Cystine and Selenium with patented-release technology.
-
Product Baroscon Double action syspension
Relieve acidreflux, heartburn, indigestion, excuess stomach acid

-
Product SiderAL® Pharmanutra
SiderAL® is the range of supplements with Sucrosomial® Iron, responding to dietary deficiencies and increased iron needs.
Sucrosomial® Technology is used to treat the iron contained in SiderAL® products, making them more resistant to the gastric environment and easier to absorb, avoiding th...
-
Product VITALDOSE® PHARMACEUTICAL GRADE ETHYLENE VINYL ACETATE (EVA)
VitalDose® EVA copolymer is a technology platform for reliable sustained release performance in indications requiring long-acting drug delivery. Common applications of this ethylene vinyl acetate pharmaceutical grade copolymer include subcutaneous and ophthalmic implants and intravaginal inserts. Ther...
-
Product Bactorinol®
Bactorinol® nasal drops contains Winterized Lentisk Oil a novel ingredient with antibacterial activity, able to disrupt bacterial biofilms to eradicate biofilm-related infections of the upper respiratory tract.
To be used alone or as add-on therapy to antibiotic therapies.
-
Product Sun Screen Color Velvet Face SPF 50+
A velvety tinted, transparent facial sunscreen that melts into the skin. The cream provides very high protection from the sun and an even colour tone. The tint covers discolouration, scars and skin imperfections. While the revolutionary Second Skin Technology creates the feeling of an invisible 'se...
-
Product KARTILIN cream
Kartilin cream contains active ingredients: glucosamine sulfate, chondroitin, MSM (Methylsulfonylmethane), collagen hydrolysate, aloe vera and ginger extract.
Aloe vera which is very effective in treating arthritis as it can reduce the amount of inflammation in the joints when applied on the are...
-
Product Paclitaxel 6 mg/ml concentrate for solution for infusion
Paclitaxel Injection is used to treat:
Ovarian cancer:
• as first therapy (after initial surgery in combination with the platinum-containing medicine cisplatin).
• after standard platinum-containing medicines have been tried but did not work.
...
-
Product Brudy Pio
Product for glaucoma pathologies (Ophthalmology). The product has been tested in different Clinical Trials, where it shows an improvement of the dry eye symptoms made by the prostaglandins, reduction of the intraocullar eye preassure. The last Clinical Trial, published in 2023, showed a significant improve...
-
Product Simethicone 80/240/500 mg
Type: Medical device
Pharmaceutical form: Soft-gel Capsules
Package: 30, 15 capsul...
-
Product ALLERGIKA® Facial Cream MED
ALLERGIKA® Facial Cream MED for the treatment of facial eczema! The perfect combination with ALLERGIKA®-Eyelid Cream MED for the treatment (duo-therapy) of all kind of facial eczema with high efficacy and compliance.
• The only lipophilic, cortisone-free medical device for the ...
-
Product Chorionic Gonadotrophin;Human Choroinic Gonadotrophin;HCG
Source: Extracted from the urine of healthy pregnant womenFunction and use: For women, it can promote the maturation and ovulation of follicles, and maintain the function of the corpus luteum. For men, it can stimulate the development of Leydig cells, increase androgen secretion, promote testicular descent...
-
Product Magnesium Diasporal® pro DEPOT muscles and bones
Magnesium Diasporal® pro DEPOT muscles and bones is a high-dose combination of magnesium plus vitamins D3 and K2. The patented dual-phase tablets have a fast and slow release: In the short-term phase, immediately active magnesium and vitamin D3 are rapidly released. In the long-term phase, long-term magnes...
-
Product Solidon Chlorpromazine 100mg tab
Chlorpromazine is a medication used to manage and treat schizophrenia, bipolar disorder, and acute psychosis. It is a member of the typical antipsychotics or neuroleptic medication category, also known as first-generation antipsychotics.
-
Product Easyfishoil Plus
EasyFishoil Plus
For children that need an easy way to boost their Omega 3 levels; contains 639 mg Omega 3 in one single delicious easyChew

-
Product JointCure
Intensive formulas of hudrolyzed collagen, silica and HA, fortified with Mg and many vitamins and minerals, produced in • 30 or20 Oral vials or • Sachets or • Powder 350gr. in Jar
for one month supply
-
Product Drug Products / BD & Licensing
For more than 30 years, Midas is actively providing licenses to market finished pharmaceutical products to its customers in an ever extending international market place. In addition to a range of differentiated finished drug products we offer projects for licensing or acquisition from our proprietary a...
-
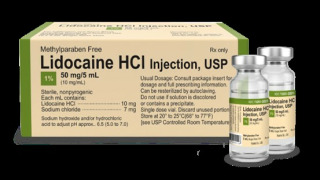
Product 1% Lidocaine HCl Injection, USP
https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=212821
-
Product Intermediate Bulk Containers for handling powders and granules.
IDEX India Pvt. Ltd offers wide range of powders & granules machinery which includes intermediate bulk containers. Features: it is designed to be filled and emptied quickly and cleaned off-line, the ibc container enables all the manufacturing processes to happen simultaneously for optimum production effici...
-
Product BAUSCH Consumables Ready to Use IV Bags
BAUSCH Consumables a division of BAUSCH Advanced Technology Group now offers “Ready to Use” IV Bags. • Volumes 50 ml - 1 L • Single or Dual Chamber • Pre-Printing on Bags • Multilayer Polypropylene Based Film • Sterile • Easy Twist Off Ports • Custom Sizes ...
-
Product Diclofenac Sodium Injection
Non-steroidal anti-inflammatory drug (NSAID) used for pain management
-
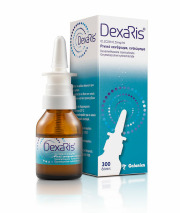
Product DEXARIS
Active ingredients: Dexamethasone isonicotinate/ Oxymetazoline hydrochlorideStrength: 0,2028 mg+0,5 mg / ml
Pharmaceutical form: Nasal spray 15 ml
Therapeutic indications:
Allergic rhinitis (hay fever, vasomotor rhinitis), acute rhinitis and rhinopharyngitis, sinusitis and nas...
-
Product Levetiracetam Injection
100 mg/ ml Intravenous Levetiracetam injection to treat & control seizures
-
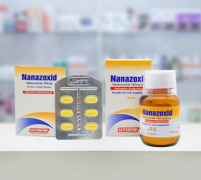
Product Nanazoxid
Active ingredient : Nitazoxanide Tablet 500 mg Suspension 100 mg/5ml
Dosage form :
Twice daily for 3 days
less than 1 year 7.5 mg/kg Suspension qq...
-
Product Anginal® Mouth Spray with Sage
Dr. Müller Pharma provides wide range of products which includes anginal® mouth spray with sage. Anginal mouth sprays contain extracts of popular herbs.
Function: for the care of the mouth mucosa. It alleviates the mucosa of throat and oral cavity. The spray protects the oral cavity, keeps it in goo...
-
Product AQaffron
The only natural adjuvant therapy to antidepressant with proven therapeutic results comparable to pharmaceutical solutions. No side effects. AQaffron is an extremely effective antidepressant aid, demonstrated by our multiple clinical studies on the finished formulation.
-
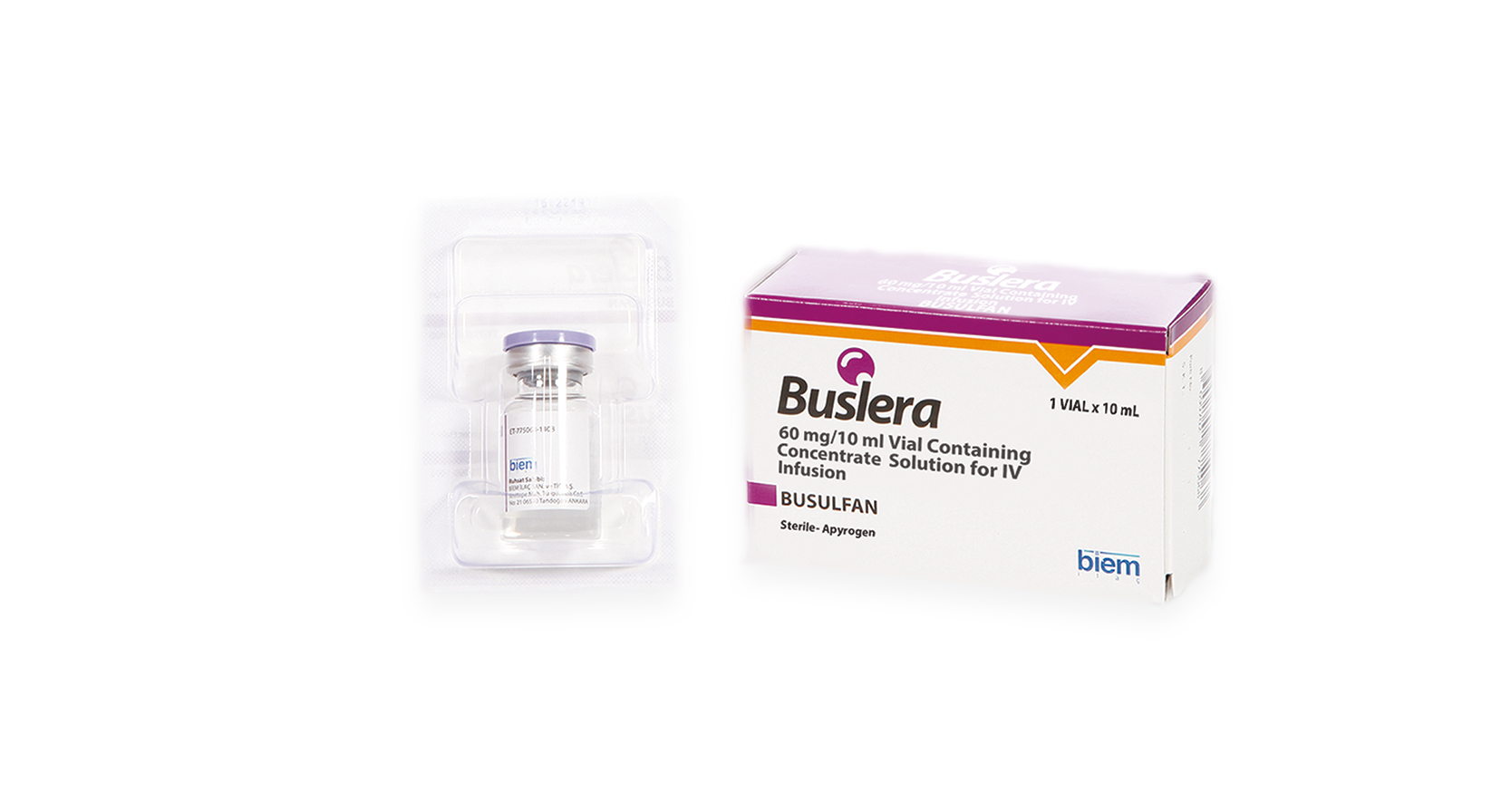
Product Buslera (Busulfan)
BUSLERA 60mg/ 10ml Vial Containing Concentrated Solution For IV Infusion
BUSLERA belongs to “alkylating agents” group of medicines, contains busulfan as active ingredient and used to clear away present bone-morrow before bone-marrow transplations.
-
Product Drug Product
At Recipharm, we offer drug product development services for all common dosage forms. Our team can manage the complexity of your project, helping you find the best solution whether you are looking for development support to take your product to first-in-human (FIH) studies or to advance your product to mar...
-
Product HydraMed®
Farmigea S.P.A. offers wide range of pharmaceutical products which includes HydraMed®. HydraMed® is an ophthalmic solution with TS-Polysaccharide (TSP®) and high-molecular weight Sodium Hyaluronate, obtained by biotechnological synthesis. The combination of these two substances gives a ...
-
Product Lozenges - Flurbiprofen
• Active ingredient (s): 8.75 mg Flurbiprofen Flurbiprofen belongs to the group of medicines known as non-steroidal anti-inflammatory drugs (NSAIDs) which are used to relive pain and inflammation. Flurbiprofen lozenges are used to reliev a painful or swollen sore throat. • Flavours (2): Mint •...
-
Product Calcium Citrate
Calcium Citrate is the best absorbed form of Calcium salt for the body. A requirement for a number of physiological requirements like development & maintenance of bones, teeth, muscle contraction, nerve condition, neurotransmission, blood clotting, regulating the heart beat & fluid balance within t...
-
Product Filgrastim
Filgrastim, Neupogen, G-CSF, Indicated to neutropenia, Formulated bulk liquid, l Injection (ampoule/PFS/vial) 75µg/unit, 150µg/unit, 300µg/unit, 450µg/unit

-
Product Bifizen®Kids
BIFIZEN® Kids is a natural, probiotic-based solution for Kids mental and cognitive
health.

-
Product Diclofenac Epolamine Medicated Plaster
MIAT has developed and registered (Hybrid legal basis) in some European countries a medicated plaster based on Diclofenc Epolamine for the symptomatic local treatment of pain and inflammation of rheumatic or traumatic nature of joints, muscles, tendons and ligaments. The product is available only through i...
-
Product EUDRACAP Functional ready-to-fill capsules
EUDRACAPTM functional ready-to-fill capsules can optimize your release profile, protect your active ingredients, and help accelerate your speed to market. EUDRACAP™ is ideal for use with active ingredients which are sensitive to heat, moisture or gastric acid to optimize absorption and avoid premature ...
-
Product ARFEN
ACTIVE PRINCIPLE INGREDIENT: ibuprofen lysinate. INDICATIONS: NSAID. Chronic symptomatic treatment of rheumatoid arthritis and osteoarthritis. PHARMACEUTICAL FORM - DOSAGE: injectable ampoules 3ml - 600mg PACKAGE: 6 vials/box
-
Product Weight loss - Obesity
Appetite Suppressant, Appetite Suppressant Forte, Fat Blocker, Flat Belly, Fat & Sugar & Carb Blocker
-
Product Tadalafil 2.5 - 5 - 10 - 20 mg - ODT in blister
Urology.ODT delivery form.
Available for outlicensing worldwide.
Contact us for more info.
-
Product Refcon Advance 100 mg + 20 mg/ml 200ml Oral Solution
Sodium Alginate / Potassium Bicarbonate - Reflux suppressant
-
Product Nutrisprint Multivitaminico
Ready intake of Vitamins and Minerals. Range of application
• in case of vitamin deficiencies (treatment and prevention)
• in case of increased need for vitamins and minerals
• in case of convalescence and fatigue
Classic complete multivitamin in combination with mineral salt...
-
Product Protofer
Dietary supplement of Iron, useful to fill nutritional deficiencies or increased needs of this nutrient.
INGREDIENTSWater, Ferrous sulphate, acidity regulator: Sodium lactate sol. 60%, colouring: caramel (may contain sulphur dioxide), Aroma, preservative: Potassium sorbate. Acidity regulato...
-
Product Basalin®(insulin glargine injection)
Basalin® is an insulin glargine injection developed and produced independently by Gan & Lee Pharmaceuticals. It was launched in China in 2005 and has been approved in almost 20 countries globally. As a long-acting biosimilar insulin, Basalin® is able to stablely control human blood sugar within 24...
-
Product Products in Private Label
We offer customers a complete range of medical devices for nasal and eye care, ready for customisation with the graphics and brand of the distributor. They are CE-marked and can be sold in EU and non-EU countries, once they have been registered locally.
-
Product Drug Product
Whether you’re developing a new chemical entity or a first-to-market generic, precise dosing and drug delivery is vital for success. Regulatory agencies and clinicians want to see well-characterized, reliable doses with good bioavailability. Patients/consumers often favor a controlled release dosage form, ...
-
Product cGMP Manufacturing Services
Our Operations team has extensive experience in cGMP clinical drug product manufacturing. Our state-of-the-art facilities and cGMP compliant systems are specifically designed for quick-to-clinic operations. Eurofins CDMO’s team of Engineers, Technology Transfer specialists, and Scientists specialize in pro...
-
Product Generic (Irbesartan + HCTZ)
- Active substance(s): Irbesartan + HCTZ;
- Pharmaceutical form(s): Tablets
- Strength(s): 150 + 12,5 mg; 300 + 12,5 mg; 300 + 25 mg
-
Product EU GMP Oral Solid Dosage List
LODAAT's Oral Solid Dosages are EU-GMP facility approved and are available in innovations such as ODT, Sachets, and Extended Release. Our facilities handle all testing, production and full packaging capabilities.
-
Product Corneial gocce 10 ml - eye drops
Corneial® gocce is a Medical Device class IIa sterile, an adjuvant solution for the prevention of red and tired eyes. Eliminate inflammation and promote re-epithelialization. Protects the corneal epithelim and improves ocular disconfort. Suitable for anyone suffering from dry eyes, poor tear form...
-
Product AteroLip 5D
Aterolip 5D is a complex of plant-based substances, vitamins and minerals that are suitable for the diabetic care. It maintains normal blood glucose and cholesterol levels and reduces deficiency of vitamins and minerals. • Monakolin K helps to maintain normal blood cholesterol levels. ...
-
Product Optiserum sterile eye wash 5ml single dose 10
Laboratoires Gilbert provides wide range of health products which includes optiserum sterile eye wash 5ml single dose 12. Brand name: physiodose and a-earwax. It is a sterile solution in single-dose, perfectly suited to the eye hygiene. Its composition ensures identical to that of tears ph. The cornflower ...
-
Product Amoxicillin and Clavulanate Potassium Tablet
Reyoung Pharmaceutical Co. Ltd. is one of the leading manufacturer and distributor of pharmaceutical products.
It has 31 workshops, which can produce ten categories and more than 400 specifications of pharmaceutical products including tablets, capsules, granules,...
-
Product Pharmaceutical formulations
Summit Pharmaceuticals Europe provides strategic solutions for the generic markets thanks to the collaboration with selected partners which are specialized in unique technologies and with specific expertise in the manufacturing of the drug products and in the regulatory service.

-
Product Livosil 22,5 mg/140 Mg Hard Capsules
Livosil - a herbal remedy that protects the liver and restores its cells after chemical drugs, fatty foods, alcohol and viral infections. The active ingredient - silymarin inhibits the effect of liver hepatotoxic substances, promotes regeneration of damaged liver cells, acts as antioxidant by increas...
-
Product Desflurane for inhalation
Desflurane is a highly fluorinated methyl ethyl ether used for maintenance of general anesthesia.
Strength: 240ml
It is approved by NMPA.
It is approved by US-FDA and EU.

-
Product ICPA PRODUCT BASKET
DERMATOLOGY : Lidocaine+Prilocaine patch & Cream, Mupirocin Ointment, Ketoconzaole Shampoo, Betamethasone cream, Fusidic acid cream, Diaper rash cream and many more..
NASAL & THROAT SPRAY: Mometasone Spray, Budesonide Spray, Fluticasone Spray and many more...
ORAL CARE: Lido...
-
Product Electrorush
Electrorush is a flavored and also with juice , ready-to-drink Oral Re-hydration Solution that provides children and adults with the appropriate amount of electrolytes and fluids they need during mild to moderate dehydration. Concept: Instead of mixing the ORS powder in water, this is...
-
Product DIKIROGEN ZERO
Food supplement recommended to counteract the metabolic and reproductive alterations related to the syndrome of insulin resistance, such as PCOS, infertility, gestational diabetes, dyslipidemia. (Myo-Inositol, D-Chiro-Inositol, Folic Acid, Manganese). Sugar free, lactose free, gluten free, suitable for veg...
-
Product ORS - Electrona
Electrona - ORS - Oral Rehydration Solution / Oral Electrolyte.Electrona is available as tetra pack of 200ml easy and practical for use. It is available in two flavors: Orange and Green Apple. Electrona / ORS is new in Lagap portfolio.
-
Product Tocovid SupraBio
Mixed Tocotrienols; bio-optimum absorption; patented product. Contact us for more information.
-
Product RIVAROXABAN FDF
Medichem S.A. offers wide range of pharmaceutical APIs and FDF which includes Rivaroxaban. Indication: Antithrombotic. Contact us for more information.
-
Product Morphine Sulphate
Morphine Sulphate Injections -1mg/ml in 2ml Ampoule, 10mg/15mg/20mg/30mg per ml in 1ml AmpouleMorphine Sulphate Tablets - 10mg/15mg/20mg/30mg/60mg/100mg Tablet Morphine Sulphate Prolonged Release Tablets: 10mg/20mg/30mg/60mg/100mg/120mg per tablet
-
Product Move Help
Aquamin minerals - 100% pure calcified red seaweed of the genus Lithothamnion, contains:calcium, which contributes to the normal functioning of muscles and bonesmagnesium, which also has a beneficial effect on bones and contributes to the reduction of tiredness and fatigue Boswelliasupports the muscul...
-
Product General, β-Lactum / Cephalosporin Products (Dedicated manufacturing area), Formulations in form of Tablets, Capsules, Liquid-orals, Ointments, Creams, Lotions
Bharat Parenteral Ltd. located at village Haripura of Savli Taluka is 24 km away from Vadodara, a major Industrial hub of Gujarat. It has state of the art manufacturing facility for General, β-Lactum / Cephalosporin Products (Dedicated manufacturing area), Formulations in form of Tablets, Capsules...
-
Product Doripenem
Doripenem is an exceptional antibiotic which is effective against complicated infections which offers broad - spectrum coverage and reliable treatment outcomes.
Doripenem is an indispensable tool for healthcare providers that combat a wide array of infectious diseases.
-
Product Amoxicillin Capsule 500mg AMOXIL BP
Nanjing sino pharmaceutical ltd. Provides wide range of products which includes amoxicillin capsule 500mg amoxil bp. Indication:treating infections caused by certain bacteria. It is also used with other medicines to treat helicobacter pylori infection and ulcers of the small intestines. Contact us for more...
-
Product Swiss Garnier Group of Companies
Development and ManufacturingContract Manufacturing
Technology Transfer and Site Transfer
Co Development
In Licensing & Out Licensing
Out Sourcing
-
Product Alphaketoanalogue
Ravenbhel Healthcare Pvt. Ltd. Offers a wide range of products which includes Alphaketoanalogue Tablets.
-
Product INJECTABLES
Requiring injectables.EU-GMP is a must.
EU dossier is a must.
Licensing and supply model or distributorship.
-
Product Ciprofloxacin Tablets
This product is a film coated sheet, after removing the coating will appear white to yellowish. For all kinds of infections caused by sensitive bacteria.Dosage: 500mg Tablets

-
Product Melatonin
1. We are manufacturing Melatonin in our facility Swati Spentose Pvt. Ltd. which is a USFDA-inspected, EU-GMP Certified, WHO GMP certified, COFEPRIS Mexico and Korean MFDS-approved facility.
2. We can supply Melatonin USP, BP as well as Melatonin EP
3. We have Written...
-
Product Omeprazole Pellets
Anzen Exports provides wide range of pharmaceutical products which includes Omeprazole. Contact us for more information.
-
Product EZETIMIBE + ROSUVASTATIN
10mg/5mg, 10mg/10mg, 10mg/15mg, 10mg/20mg, 10mg/30mg, 10mg/40mg tablets, Rosuvastatin/ezetimibe combines two lipid-lowering agents: rosuvastatin, an HMG-CoA reductase inhibitor (i.e. statin) with particularly strong inhibitory effects on hepatic cholesterol synthesis, and ezetimibe, which inhibits the...
-
Product Bortezomib, Bortezomib for Injection (3.5 mg/vial)
Hainan Shuangcheng Pharmaceuticals Co. manufactures apis and generic finished dosage products which include Bortezomib and Bortezomib for Injection (3.5 mg/vial). Contact us for more information.
-
Product Metopimazine 7.5mg ODT
Venipharm. develops and registers a wide range of generics dossiers which includes Metopimazine 7.5mg ODT . Product is available for export markets.
Please contact us for more information.
-
Product Technology transfer and Plant approvals from EU GMP/USFDA/PICS/EA EU etc.
Technology Transfer-Experienced team of scientist visit the plant for preparation and training of manufacturing team to manufacture prototype and exhibit batches, analytical testing and stability studies
Plant Approvals-Experienced auditors and QPs perform the gap assessment and help the manufac...
-
Product Vancomycin Injection USP/BP
It belongs to tablet category. finiContact us for more information.
-
Product Latanoprost
Latanoprost is a type of prostaglandin. It is indicated for antiglaucoma treatment. Originator: Pfizer. Brand: Xalatan. Contact us for more information.
-
Product Effervescent Tablets
As the expert in user-friendly dosage forms we can make effervescent tablets that have a pleasant taste, even if they contain bitter or difficult-to-process APIs, ensuring they leave neither residue nor foam when they dissolve. The composition of our effervescent tablets helps to speed up the absorption of...
-
Product Out Licensing Dossiers
• Out Licensing • Technology Transfer • Co-Development / Profit Sharing with •
Technology Partners
API partners
Marketing Partners
CMO Partners

-
Product Octreotide Acetate LAR Injection
Octreotide acetate for acromegaly, carcinoid tumors and VIPoma
-
Product Aseptic Processing / Syringes with solid implants
Harro Höfliger Verpackungsmachinen provides wide range of products which includes aseptic processing / syringes with solid implants. It is to equip a syringe with the solid implant, the syringe barrel is disassembled. After completion of the product, it is labeled and subsequently fitted with a protective...-comp272211.jpg)
-
Product Close Loop Granulation Line - 2 Bar and 12 Bar Pressure Shock Resistant
1. Complete close loop dust-free operation right from sifter till blender bins.2. Both 2-bar and 12-bar forms are equipped with a pressure shock-resistant design
3. In-house manufacturing of all major types of equipment
4. Through-the-wall design can be offered to reduce the footprint
5. Bat...
-
Product Melatonin Products
Mouth & Throat Spray with Melatonin
Lozenges with Melatonin
100% free from preservatives
-
Product Ampicillin + Sulbactam Sterile Powder for Injection
Pharmaceutical form: Powder for Solution for Injection or InfusionDCI: Ampicillin with Sulbactam
Therapeutical class: Penicillin Beta Lactamase Inhibitor
Packaging: Box x 1 vial / box x 10 vials / box x 25 vials
Contact us for more information.
-
Product Licensing for Partners
PharmaMatch represents companies from all over the world and we have always shown ourselves to be a reliable and trustworthy partner. Our sales team has an excellent network and they are continually assessing the market per product to find the best match. Our partners can expect our Sales team to appro...
-
Product MyInfla
Pharmascience Inc provides a wide range of key products which includes MyInfla. Form: colchicine extended-release tablets. MYINFLA is indicated for the reduction of atherothrombotic events in adult patients with existing coronary artery disease, in addition to standard therapies...-comp241600.jpg)
-
Product Finished Products
Egis Pharmaceuticals PLC provides a wide range of products which includes finished products. Contact us for more information.
-
Product Modular Cleanrooms
AASTHA Cleanroom Partition is designed according to GMP requirements and to provide a clean and reliable environment for either new or existing facilities. We provide excellent flush surface to prevent possibility of contamination, microbial and fungal growth. The high quality components allow a high ...
-
Product Esomeprazole Sodium Sterile (Lyophiolized)
RAL is the leading manufacturers of esomeprazole sodium sterile. Commercial production is carried out in units operating as per cGMP standards and equipped with at par quality equipment and utilities. A well-established cleaning and validation procedures that ensure high quality of products by avoiding...
-
Product Biocollagen
Normon offers a wide range of dental products which includes biocollagen. Contact us for more information.
-
Product Alendronate + Cholecalciferol
Pharmaceutical form: Tablets Therapeutic category: Postmenopausal osteoporosis.
Strengths: 70mg/2800IU & 70mg/5600IU
Contact us for more information.
Products which are subject to patent protection are currently not offered or made available in Germany or in countries w...
-
Product Amrizole (infusion)
Pharco corporation offers a wide range of generic drugs which includes amrizole (infusion). Contact us for more information.
-
Product COMPANY PROFILE
Please find here our CP with all our patented products available for the export.
-
Product Caspofungin
Galenicum offers a wide range of pharmaceutical products like Caspofungin. Contact us for more details
-
Product Prefilled Syringes
Delpharm group offers pharmaceutical services which includes glass prefilled syringes. Capsules. Contact us for more information.
-
Product Disease Activity Models/Tissue Culture
MedPharm has developed proprietary models relevant for major disease areas such as psoriasis, atopic dermatitis, onychomycosis and continue to develop new ones through our dedicated innovation group. These sophisticated ex vivo experiments provide additional confidence to clients and their in...
-
Product Oncology Injectables, Oral Solid Dosage Forms & APIs
Quality products manufactured in highly compliant facility for registration and sales in highly regulated markets.
-
Product CMO Services
CMO services could be offered for Oral Solid forms - especially for Oncology products - in PharOS manufacturing plant in Malta.
-
Product Value Added Dasatinib
Value added Dasatinib (Teaser attached) • Dasatinib is a targeted therapy used to treat certain cases of chronic myelogenous leukemia (CML) and acute lymphoblastic leukemia (ALL) • Current available products on the market are not completely absorbed by those patients ...
-
Product Out-licensing
Medis is an established provider of dossiers and products in all areas of the world with launches and marketing authorizations in over 140 countries to date.
-
Product Multi-enzyme Digestive Tablets
Digestive disorders has become a common occurrence in today's times due to unhealthy and stressful lifestyle. This hampers overall health and well-being subsequently leading to various related disorders. Multi-enzyme Digestive Tablets consist of essential Enzymes which help in digestion of a broad range of...
-
Product Sunglow Pharmaceuticalss Pvt. Ltd.
We offer finished dosage forms including coated, uncoated, bilayered, capsule and sachet products in the following therapeutic segments.
· Anti Diabetic
· Anti Neoplastics
· &n...
-
Product AlgaeCal
Generex Pharmassist Pvt. Ltd provides wide range of nutraceutical products which includes algaecal. It is the world's first plant source of calcium introduced by algaecal inc., canada. Among the various calcium sources available in the market today, algaecal provides several product differentiation and adv...
-
Product Human Histaglobulin
The main component of this preparation is human histaglobulin, of which the human immunoglobulin is originated from human plasma from healthy donors. The manufacturing process contains a step of low pH incubation for viral inactivation. It contains suitable amounts of sodium thiosulfate and glucose a...
-
Product Fudesix (Furosemide Oral Solution 20mg/5ml, 50mg/5ml Therapeutic indications)
Furosemide is indicated in conditions requiring prompt diuresis, including cardiac, pulmonary, hepatic and renal oedema, peripheral oedema due to mechanical obstruction or venous insufficiency and hypertension in children.
It is also indicated for the maintenance therapy of mild oedema of any origi...
-
Product Contraception.
Cyndea Pharma S.L provides wide range of woman's health related products (hormones) which includes contraceptives. Contact us for more information.
-
Product Metformin HCl 500 mg SR + Pioglitazone HCl 15/30 mg Tablets
Metformin HCl 500 mg SR + Pioglitazone HCl 15/30 mg Tablets Non DMF
Glucophage® Metformin HCl XR Tablets 500 mg (BMS, US) + Actos® Pioglitazone HCl Tablets 30 mg (Takeda, US)
Find your finished dosage forms suppliers online: our directory lists nearly 500 suppliers offering 2,500+ products that range from creams and capsules to powders, sprays, suspensions and more.
Finished Dosage Formulation
A Finished Dosage Form is a finished drug product resulting from Finished Dosage Formulation (FDF), the various processes involved in production such as manufacturing, testing, and subsequent approval for consumption and delivery to the public.
Active Pharmaceutical Ingredients (API’s), responsible for the results the user gets after taking the dosage forms, are involved in the FDF along with inactive ingredients (excipients) which serve as a medium for the active ingredients to function.
FDF is a critical stage in the product lifecycle and can only be achieved if the manufacturing facility of a pharmaceutical company or Contract Manufacturing Organization is up to a high-end standard. This is a major reason why some companies are into out-licensing since they don’t have the standard manufacturing facility to produce the dosage forms.
Processes Involved in Finished Dosage Formulation Development
The processes involved here are quite complex. Analysis comes first involving the active pharmaceutical ingredients and excipients. A compatibility study is also done between them.
Next is the formulation development which includes the definition of quantitative and qualitative formulas, formulating the manufacturing method, and establishment of in-process controls.
The next process is the main manufacturing process, which then proceeds to quality control, clinical trials, stability studies and manufacturing regulations before the drug product is transferred to the client if it’s been conducted by a pharmaceutical contract manufacturing organization.
What are the Benefits of Outsourcing Finished Dosage Formulation Development?
Formulation development is an integral process for any commercial manufacturing pharma company and even for private purposes in the development of solid dosage forms. This is why outsourcing always comes in handy because the pharmaceutical contract manufacturing firms are usually more equipped with state-of-the-art manufacturing equipment.
They also ensure that the drug product meets the required industry benchmark using the right method development and standard clinical trial materials to ensure there are no loopholes.
Overall, outsourcing ensures that the dosage form is up to the best standard and is duly regulated.
Growth in Finished Dosage Formulations Market
The key factors that are driving trends in the finished dosage formulation market are:
• Access to cutting-edge technological resources in the global pharmaceutical industry.
• An increased need to outsource drug development and pharmaceutical contract manufacturing.
• The increasing contract manufacturing market share of the industry.
Laws and Regulations for the Manufacture of Finished Dosage Formulation
The US FDA’s Current Good Manufacturing Practice regulations require three successful full-scale batches of finished dosage formulation. These three validation batches are essential to ensure that process design and development studies are up to the required standard for active pharmaceutical ingredients, excipients, and drug production generally. GMP compliance is vital to acquire a license to manufacture finished dosage formulations like soft gelatin capsules.
Finished Dosage Formulation
A Finished Dosage Form is a finished drug product resulting from Finished Dosage Formulation (FDF), the various processes involved in production such as manufacturing, testing, and subsequent approval for consumption and delivery to the public.
Active Pharmaceutical Ingredients (API’s), responsible for the results the user gets after taking the dosage forms, are involved in the FDF along with inactive ingredients (excipients) which serve as a medium for the active ingredients to function.
FDF is a critical stage in the product lifecycle and can only be achieved if the manufacturing facility of a pharmaceutical company or Contract Manufacturing Organization is up to a high-end standard. This is a major reason why some companies are into out-licensing since they don’t have the standard manufacturing facility to produce the dosage forms.
Processes Involved in Finished Dosage Formulation Development
The processes involved here are quite complex. Analysis comes first involving the active pharmaceutical ingredients and excipients. A compatibility study is also done between them.
Next is the formulation development which includes the definition of quantitative and qualitative formulas, formulating the manufacturing method, and establishment of in-process controls.
The next process is the main manufacturing process, which then proceeds to quality control, clinical trials, stability studies and manufacturing regulations before the drug product is transferred to the client if it’s been conducted by a pharmaceutical contract manufacturing organization.
What are the Benefits of Outsourcing Finished Dosage Formulation Development?
Formulation development is an integral process for any commercial manufacturing pharma company and even for private purposes in the development of solid dosage forms. This is why outsourcing always comes in handy because the pharmaceutical contract manufacturing firms are usually more equipped with state-of-the-art manufacturing equipment.
They also ensure that the drug product meets the required industry benchmark using the right method development and standard clinical trial materials to ensure there are no loopholes.
Overall, outsourcing ensures that the dosage form is up to the best standard and is duly regulated.
Growth in Finished Dosage Formulations Market
The key factors that are driving trends in the finished dosage formulation market are:
• Access to cutting-edge technological resources in the global pharmaceutical industry.
• An increased need to outsource drug development and pharmaceutical contract manufacturing.
• The increasing contract manufacturing market share of the industry.
Laws and Regulations for the Manufacture of Finished Dosage Formulation
The US FDA’s Current Good Manufacturing Practice regulations require three successful full-scale batches of finished dosage formulation. These three validation batches are essential to ensure that process design and development studies are up to the required standard for active pharmaceutical ingredients, excipients, and drug production generally. GMP compliance is vital to acquire a license to manufacture finished dosage formulations like soft gelatin capsules.
References
Upcoming Events
-
-
-
CPHI & PMEC India 2024
India Expo Centre, Greater Noida, Delhi NCR
26 Nov 2024 - 28 Nov 2024
Pharmaceutical Industry Webinars
-
Webinar Fragment-Based Oligonucleotide and Oligopeptide Synthesis
-
30th Jul 2023
-
4pm CET / 10am EST
-
-
Webinar GMP Rationale for Sterile High-Potency/Toxic Pharmaceuticals
-
18th June 2024
-
4pm CET / 10am EST
-
-
Webinar Unlocking Opportunities in the Growing Pharma Landscape of The Middle East
-
5th June 2024
-
3pm CET / 9am EST
-
-
Webinar Exploring Technological Trends in the Future of Pharmaceutical Manufacturing
-
23rd May 2024
-
4pm CET / 10am EST
-
-
Webinar Achieving Manufacturing Excellence Through Digital Transformation
-
16th April 2024
-
4pm CET / 10am EST
-
-
Webinar Made in Africa: What’s Driving Pharma Manufacturing
-
28th March 2024
-
4pm CET / 10am EST
-
-
Webinar Case Study: Risk Management for Annex 1 Sterile Production EMS
-
28th February 2024
-
4pm CET / 10am EST
-
-
Webinar Innovative Strategies for B2B Pharma Marketeers: Driving Value through Content
-
20th February 2024
-
4pm CET / 10am EST
-
-
Webinar Revolutionizing Pharma: Data and AI Unleashed
-
18th January 2024
-
4pm CET / 10am EST
-
-
Webinar Optimal Temperature: Elevating Biologics Cold Chain Excellence
-
16th January 2024
-
4pm CET / 10am EST
-
-
Webinar Market Outlook – The Biggest Pharma Trends of 2024
-
12th December 2023
-
4pm CET / 10am EST
-
-
Webinar The Next Frontier – Emerging Opportunities in the LATAM Pharma Market
-
21st November 2023
-
4pm CET / 10am EST
-
-
Webinar Vistamaxx™ MED - imagine the possibilities for healthcare product performance
-
10th October, 2023
-
4pm CET / 10am EST
-
-
Webinar Co-processing: A Multifaceted Approach for Enhancing Density & Powder Flow
-
19th September 2023
-
4pm CET / 10am EST
-
-
Webinar The Outlook for Cell & Gene Therapy Manufacturing
-
28th June 2023
-
4pm CET / 10am EST
-
-
Webinar Contract Packaging Outlook: Growth Trends within the Commercial Packaging Sector
-
25th May 2023
-
4pm CET / 10am EST
-
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance









































































































-comp241600.jpg)








































































































.png)
.jpg)

%2010.png)







.jpg)









.jpg)




































































































.png)

.png)



.jpg)