- Home
- Finished Dosage Forms (Products)
- Injectables
CPHI Online is the largest global marketplace in the pharma ingredients industry
-
Products551,744
-
Companies7,781
-
Articles11,636
-
Events8
-
Webinars342
Injectables
Injectables Companies (202)
Injectables News
-
News Latest updates for semaglutide: reduction of renal failure risk
Recent data presented at the 2024 European Society for Cardiology (ESC) conference analysed Novo Nordisk’s Ozempic (semaglutide) and its effectiveness in the management of chronic kidney disease (CKD). This follows previous analysis on semaglutid...4 Sep 2024 -
News Eisai Alzheimer’s drug authorised in UK but still faces obstacles
In partnership with BioArctic AB, pharmaceutical company Eisai has been granted Marketing Authorisation by the Medicines and Healthcare products Regulatory Agency (MHRA) for its Alzheimer’s disease drug product Leqembi.22 Aug 2024 -
News Novel oral Type 1 diabetes drug gains US FDA IND designation
A University of Alabama at Birmingham startup has gained FDA clearance for Investigational New Drug clinical trials for an oral Type 1 diabetes drug, a milestone for diabetes treatment.1 Aug 2024 -
News Second obesity drug candidate from Roche shows early trial success
Following from its purchase of Carmot Therapeutics, Roche have announced positive early-stage trial success for its second obesity drug candidate acquired from Carmot Therapeutics.17 Jul 2024 -
News Reworked once-daily weight-loss pill from Pfizer to progress
American pharmaceutical giant Pfizer have continued with a reworked version of their weight-loss pill danuglipron, progressing with a once-daily version of the pill.12 Jul 2024 -
News This week in GLP-1 drug headlines: Manufacturing investment and new market launches
As drugmakers race to put their own GLP-1 drug products forward as the next biggest thing in weight-loss, current products are making numerous headlines this week with a number of new developments in their commercialisation and approval. Discover the l...26 Jun 2024 -
News Novo Nordisk launches 'Power of Wegovy' national campaign
Danish drugmaker Novo Nordisk have launched a new national campaign – The Power of Wegovy – that aims to educate those living with obesity on their blockbuster drug Wegovy throughout the United States.5 Jun 2024 -
News Eisai begins US FDA rolling submission for Alzheimer’s drug Leqembi
Japanese drugmaker Eisai, in partnership with Biogen, have announced that they have begun submitting data on a rolling basis to the US FDA for a marketing application of a subcutaneous injectable form of Alzheimer’s drug Leqembi.15 May 2024 -
News Novo Nordisk gains expanded approval for Wegovy to treat heart failure
Wegovy, the blockbuster weight loss drug from Danish drugmaker Novo Nordisk has now received expanded approval from the US FDA for use in the prevention of stroke and heart attacks.11 Mar 2024 -
News Wegovy® – the weight-loss drug sparking a global race to end obesity
Wegovy®, the weight loss drug from Novo Nordisk has been creating headlines since it’s early testing phases, through it’s approval in June 2021 by the US FDA and January 2022 from the European Medicines Agency, and it continues to do so...26 Feb 2024 -
News Novo Nordisk to invest $2.3 billion in French facility to address GLP-1 drug shortage
Manufacturing for the diabetes and weight-loss drugs Ozempic and Wegovy is expected to increase with a billion-dollar investment Novo Nordisk production site located in Chartres, France.29 Nov 2023 -
News CPHI Online Webinar Series – The CPO Perspective: Driving Healthcare Sustainability
Pharmaceutical contract packaging organisations (CPOs) offer organisations broader expertise in pharma packaging, specialist skills, and flexibility with time, efficiency, and costings for pharmaceutical companies lacking in-house packaging expert...1 Jun 2023 -
News Doctors Without Borders demands transparency from GSK on licensing agreements for preventative HIV treatment
GSK has signed licensed agreements with three companies to manufacture generic versions of its preventative HIV medicine for distribution in lower-income countries but calls for increased transparency is putting pressure on the drugmaker.4 Apr 2023 -
News GSK opens license for HIV preventative to increase access in lower-income countries
GSK has voluntarily opened the intellectual property rights on it's long-acting injectable HIV preventative medicine to three companies, so that they can make generic versions that will be more accessible to less-developed countries.30 Mar 2023 -
News Eli Lilly, Novo Nordisk, and Sanofi cut insulin prices by up to 78%
Insulin prices have dropped in a stark play by the three biggest makers of the product, after calls from the top of the US government.21 Mar 2023 -
News Pharmapack 2023 – Take the Start-Up innovation tour
At Pharmapack 2023 in Paris we debuted a new and improved Start-Up Hub. The Hub included several start-up companies we are looking to support and make the event more accessible.14 Mar 2023 -
News CARBOGEN AMCIS opens new liquid drug product manufacturing facility
The Switzerland-based pharmaceutical process and ingredient developer and manufacturer announced the opening of their new sterile liquid drug product manufacturing facility in France.2 Feb 2023 -
News The Biggest Drug Approvals of 2022
A carefully curated list of the most notable drug approvals of the past year, covering neurology, oncology, and COVID-19, as well as a look at the most promising drugs up for review in 2023.9 Jan 2023 -
News CPHI Frankfurt: Interview with Marcelo Cruz, Vice President, Business Development & Marketing at Tjoapack
In this series of interviews, we caught up with some of the exhibitors at CPHI Frankfurt to discover what innovations are being brought to the pharmaceutical industry this year. Here we chat with Marcelo Cruz of Tjoapack to discuss how global events ar...23 Dec 2022 -
News CPHI Frankfurt 2022: Innovator Interview – DSM Biomedical
At CPHI Frankfurt we spoke to Anne-Cecile Bayne, Global Science & Innovation Lead Pharma and Medical Nutrition, and Marc Hendriks, Vice President Strategy & Business Development, on their expertise in nitrosamines and business strategy at DSM Biomedica...23 Nov 2022
Injectables Products (500+)
-
Product Biolevox™ Tendon
Biolevox™ HA Tendon is hyaluronic acid (HA) gel especially designed for intratendinous or peritendinous injection as a double application therapy in order to alleviate the ongoing inflammation process and further recovery of the tendon functionality. HA-based gel contains 32 mg of sodium hyaluronate in 2.0...
-
Product Device Design Development & Human Factors
The Sanner Group with its Design Centers of Excellence in UK and the US (Springboard and Gilero) is a global Contract Development and Manufacturing Organization (CDMO), dedicated to developing high-quality and robust drug delivery and diagnostic devices for its customers. With over 150 engineers whose expe...
-
Product Ferric Carboxymaltose Inj/IV, Iron Isomaltoside inj/ IV, Heparin, Citicoline, Fluphenazine, Haloperidol, Levetiracetam, Iron Polymaltose Inj,L-Ornithine L-Aspartate Injections
Hematinic Injectables-Ferric Carboxymaltose Inj/IV (50 mg per ml)- treats anemia (Leads to Instant Hemoglobin rise with No Anaphylactic Shock,Highest Patient Compliance,Highest Safety Profile amongst all the Intravenous hematinic,Widely recommended for Critical Care Patients under CKD, Cardio-vascular Dise...
-
Product Aenova Sterile Fill Finish & PFS manufacturing
The Aenova Group is a premier solution partner for sterile dosage forms with 3 FDA approved facilities offering high quality injectables for human health and animal health products.
Our Sterile services include specialized capabilities for:
Beta-lactams (penicillins, cephalosporins)In...
-
Product EVER Pharma Product List
Our products include: Atosiban Cabazitaxel Cebrium(R) Cerebrolysin(R) Dacepton(R) Dexmedetomidine Docetaxel Fulvestrant MemoProve(R) Oxytocin Paclitaxel Pemetrexed Tachyben(R) Terlipressin
-
Product MEDSAMIC, Tranexamic Acid Solution for injection or infusion 100mg/ml, 250mg/5ml, 500mg/5ml
Hemostatic agent: Antifibrinolytic
-
Product Somatropin for injection USP 10 IU - Shreetropin
A human growth hormone drug for dwarf child
Shree Venkatesh International Ltd offers a wide range of pharmaceutical products, including the Somatropin for injection USP 10 IU-Somatropin.
• Use: Treatment for children with growth failure due to inadequate secretion of...
-
Product OSTENIL®
OSTENIL® is a solution containing hyaluronic acid for injection into the joint cavity.
OSTENIL® is indicated for the treatment of symptoms of osteoarthritis. It has been shown to reduce pain and stiffness in the affected joint and to improve joint function
Pre-filled syringe to relieve pain in...
-
Product ANTIBIOTIC INJECTABLES Meropenem(FOR CRITICAL CARE)
CEFEPIME FOR INJECTION | CEFTRIAXONE | CEFTAZIDIME FOR INJECTION | CEFOPERAZONE + SULBACTAM FOR INJECTION | CEFUROXIME FOR INJECTION | CEFOTAXIME FOR INJECTION | CEFTAZIDIME AVIBACTAM FOR INJECTION | CEFTRIAXONE SULBACTAM FOR INJECTION | ELORES: CEFTRIAXONE, SULBACTAM & EDTA FOR INJECTION | AZTREONAM F...
-
Product Vial Powder filling Line
• Full complete integrated production line consisting of washing machine, sterilizing & depyrogenating tunnel, liquid/micro powder dosing, filling, stoppering & cap sealing machine. • Containment system in the form of oRABS/cRABS/Isolator. • Powder filling by measures of cams/servo mot...
-
Product Grindeks FDF portfolio
Please see Grindeks up-to-date Final Dosage Form (FDF) product portfolio, including pipeline products, in the link attached. Dossiers are harmonized according to ICH and EMA requirements. https://grindeks.com/en/products/final-dosage-forms/
-
Product OSTEOARTICULAR HEALTH LINE (POLYNUCLEOTIDES HPT™/ POLYNUCLEOTIDES HPT™ AND HA GEL - 2ML)
Condrotide Class III Medical device for intra-articular use consisting of a viscoelastic solution containing Polynucleotides HPT™
40 mg/2ml of polynucleotides
Sterile, pre-filled glass syringe, for single use
Condrotide HA
Fluid gel for intr...
-
Product Easy Tear Tyvek Autoclave Bags
Easy-Tear Tyvek bags from Keystone Cleanroom Products are designed to satisfy the most stringent requirements in the sterilisation of critical pharmaceutical equipment and components. Easy-Tear Feature for easier opening and sealing No sterile cutting instruments required to open the bag Low parti...
-
Product Haemostop (Tranexamic Acid) Solution for Injection
Haemostop contains tranexamic acid which belongs to group medicines antihaemorragics; antifibrinolytics. It used on adults and children above one year of age for the prevention and treatment of bleeding due to a process that inhibits blood clotting called fibrinolysis. specific uses include: heavy pe...
-
Product Leuprolide 3-Month Implant
The active ingredient leuprorelin, like goserelin, is a synthetic peptide that can be used primarily in the treatment of hormone-dependent tumors, particularly prostate cancer. Administration of leuprorelin has indirect effects on testosterone levels in men, the lowering of which stops the growth of malign...
-
Product Pronutim Sachets
PRONUTIM is a unique Whey Protein Isolate, high in native cysteine, produced by an exclusive microfiltration and spray drying process, to ensure protein integrity and cysteine bioavailability as glutathione precursor: • HIGH PURITY, remarkable guaranteed titration ≥ 92.5%
• HIGH BIOLOGICA...
-
Product Needle Isolation Technology (NIT®)
NIT® is a safety solution that eliminates the need for manual needle attachment in cartridge-based injections. It enables cartridges to be built into autoinjectors, supporting treatments beyond the 2.25 mL fill range and lyophilized drugs requiring dual-chamber containers.
Products built with NIT ...
-
Product Amphotericin B Liposome for Injection
Indications:
Empirical therapy for presumed fungal infection in febrile, neutropenic patients;
Treatment of cryptococcal meningitis in HIV-infected patients;
Treatment of patients with Aspergillus species, Candida species and/or Cryptococcus species infections and treatment of visceral leishm...
-
Product Tramadol HCl
Grünenthal GmbH offers a selected range of products which includes Tramadol HCl, as API, bulk and/ or finished dosage form. Please contact us for more information.

-
Product PFS - HA CROSSLINKED FILLERS
Dedicated site production of innovative CROSS-LINKED HYALURONIC ACID pre-filled syringes (PFS) terminally sterilized and suitable for medical and orthopaedic applications, patented and innovative for the higher purification.
Contact us for more information.
-
Product Promovia® Hydro Balance
INJECTIONS Patented formulation featured of a mixture of sodium hyaluronate at medium and low molecular weights in concentration of 2,5% and 1% of Trehalose to extend the effectiveness and the duration of the treatment.
Promovia® Hydro Balance is available in two strengths:
Promovia®...
-
Product Formulation Development
Singota solutions offers wide range of services which includes analytical and formulation development. We create a robust method for analysis of product, whether starting from scratch or optimizing a method, and can help create a set of solutions that are customized to you, while supporting that metho...
-
Product OpHLINE - Ophthalmic Viscosurgical Devices (HA 1,4%/2%/3%)
OpHLINE is an ophthalmic viscosurgical devise (OVD) indicated as a coadjuvant during surgical procedures, involving the anterior chamber of the eye, especially in cataract surgery, including the extraction of the lens and the insertion of intraocular lenses.
OpHLINE is available in 3 models ...
-
Product Complex Sterile Formulations Contract Development
+25 Years of accumulated experience in Lipid and polymer-based formulations.
Regarding Complex Injectables, Bluepharma's focus is on Lipid-based, polymer-based, and Long-acting injectable (LAI) formulations. Bluepharma allies the scientific expertise with the technical capability to ensure the success...
-
Product Glass Ampoules
Type I Neutral Borosilicate glass Glass Ampuoles with brimfull capacities from 1 ml to 30 ml Clear and amber colour ISO Forms B, C and D, we can also customize special shapes One Point Cut (OPC), Colour Break Ring (CBR) and Score Ring (SC) With optimized opening forces. Up to three colored coded identifica...
-
Product Pharmaceutical Kitting
Over the past 30 years Tjoapack has developed significant capabilities and expertise in biotechnology packaging. This has been mostly driven by innovation in the space and a move towards self-administration and patient-centricity. At Tjoapack, we have a specially-trained team in place to tackle the challe...
-
Product CordenPharma's Early-Phase Peptide Centre of Excellence
In addition to the largest worldwide capacity of commercial SPPS peptide production up to batch sizes exceeding 400 kg (> 35 Amino Acids), and a yearly capacity of > 2 Metric Tons of Peptide, CordenPharma's multi-pronged approach to becoming the #1 peptide CDMO supplier extends to small-scale capabilities,...
-
Product Eptifibatide Injection
Strength: 20 mg/10 ml It is used for the prevention of early myocardial infarction in adults presenting with unstable angina or non-Q-wave myocardial infarction, and for platelet aggregation
-
Product Wearable Injectors - September 2023 - Issue 151
Available now to read online at: https://www.ondrugdelivery.com Contents: YpsoDose – the Clinic-Ready 10 mL Patch Injector Directions For Wearable On-Body Injector Systems And Beyond TTP Would You Like to Go Large? Key Considerations for Large-Volume Injectors DCA BD Innovation for Subcutaneous Delive...
-
Product D-Flex Injection Pen
D-Flex, the pen platform for subcutaneous self-injection. Bridging the gap between fixed and variable-dose pens, this innovative product also paves the way for connected commercial devices.
• Just one pen for many needs: Disposable pen for fixed or multiple fixed dosages • The versatile solution: Allo...
-
Product Calcitriol 1μg
1. Efficacy & Effectiveness
1) Treatment for hypocalcemia in chronic kidney dialysis patients.
2) Improvement of skeletal dysplasia by remarkable reducing elevated parathyroid hormone levels.
2. Administration & Dosage
The initial recommend...
-
Product Naprod Antineoplastic Formulation Injectable & Oral Solid , Propofol, General Lyophilised Injection General Lyophilised
#Propofol
#Gemcitabine
#Imatinib
#Bleomycin
#Irrinotecane
#Linalidomide
#Dasatinib
#Abiraterone

-
Product Blow Fill Seal
Curida offers contract manufacture of liquid pharmaceuticals in Blow Fill Seal ampoules.
Specialized in development activities and full-scale commercial manufacturing of aseptic, sterile and non-sterile fill & finish
Ampoules for small volume units: aseptic filling or terminal ...
-
Product Vancomycin Injection, Ready to Use
Vancomycin hydrochloride is a glycopeptide antibiotic active against a wide variety of Gram-positive bacteria, most important being staphylococci, such as Staphylococcus aureus and Staphylococcus epidermidis (including susceptible methicillin-resistant strains), but also active against corynebacterium, ent...
-
Product Drug Product Fill-Finish Services
Fill-Finish Capabilities (product type) and Capacity:
Sterile liquid products
Biosafety level (BSL) 1, BSL 2 (OEL >= 10 µg/m³)
Vaccines (inactivated)
Current capacity: ~40M units/year

-
Product Advanced PolyPropylene and PolyEthylene IV-bag Filling Tubes.
Shock resistant at lower temperatures
Sterilizable at 121°C
Customizable widths
Customizable thickness
Class C clean room extrusion
Class 100 filtered Air on the inside
Plastic formulation excellence ...
-
Product Eylea biosimilar vial & PFS
1.Completion of global clinical trials phase Ⅲ
2. Completion of pre-submission meetings with advanced regulatory authorities
3. Available in Vial and PFS
-
Product XANOIMAGE (Iodixanol Injection USP)
Iodixanol is a dimeric, isosmolar, nonionic, water soluble, iodinated x-ray contrast agent for intravascular administration.
Iodixanol Injection USP 320 mgI/ml :- Indicated for angiocardiography (left ventriculography and selective coronary arteriography), peripheral arteriography, visceral ar...
-
Product K-Pack™ II Needles
K-Pack™ II Needles are carefully crafted to achieve smoother injections of today and tomorrow’s parenteral medications – targeting on a less painful injection and thus on higher therapeutic adherence and compliance.
-
Product DYNO®-MILL MULTI LAB
Grinding container volume of 0.15 to 1.4 liters.
For many decades, WAB has been the undisputed specialist in milling and fine dispersion technology with its world renowned DYNO®-MILL. The stringent requirements which the finished product has to fulfil also demand the highest standards of quality and abr...
-
Product UniSafe 1 mL
A springless, passive safety device for 1mL pre-filled syringes, designed for simple assembly and use. Simple 2-step final assembly, Secure plunger prevents removal, spillage and repeat use. UniSafe 1mL has the following benefits:
User confidence with an unobscured syringe barrel for full dose visibilit...
-
Product 1% Lidocaine HCl Injection, USP
https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=212821
-
Product SMART Flexi-Q eMU-P
SMART Flexi-Q eMU-P is particularly suitable for use in long-term therapies that require frequent injections, such as multiple sclerosis, autoimmune conditions and blood disorders.
The reusable design of SMART Flexi-Q eMU-P lowers cost per injection and reduces amount of materials for stor...
-
Product Acetonitrile
A highly pure solvent used as an Excipient in Oncology Injectable formulations such as Bortezomib, Doxorubicin etc.
Specification:
USDMF
-
Product Steriles
Famar offers pharmaceutical services which includes steriles.
Contact us for more information!
-
Product BoniM Tablet/ Injection (Ibandronate 150mg/3mg)
Well-proven efficacy & safety High Improved Quality of Life Monthly, BoniM Tab.150mg Quarterly, BoniM Inj. 3mg
Boni M Injection (Ibandronate 3mg) Indication: Treatment of postmenopausal osteoporosis Administration/Dosage: 3mg every 3 months administered intravenously over a period of 15 to 30 seco...
-
Product Cephalosporins Finished Products
<Injection> Cefazolin Sodium 1g Cefmetazole Sodium 1g, 2g Cefotaxime Sodium 1g Ceftazidime Hydrate 0.5g, 1g, 2g Ceftizoxime Sodium 1g, 2g Cefoperazone Sodium 1g, 2g Cefotiam HCl 1g Ceftriaxone Sodium 1g Cefepime HCl 1g *Flomoxef Sodium 0.5g, 1g *Cefamandole Nafate 1g, 2g *Ceftaroline Fosamil 400mg...
-
Product Innovation Hub_Pharma Virtual Lab by Roquette
Discover how we can help you Roquette as a technology partner and a trusted supplier, provides scientists and researchers with a collaboration platform to beter understand and formulate with our ingredients. Innovation Hub | Roquette Pharma Solutions
-
Product Luphere Depot
Luphere Depot (Leuprorelin) is a generic specialty drug of Lupron/Leuplin. Luphere Depot is a marketed product in Korea since January 2005. Currently, Luphere Depot is under development for US and Japan.
Key Features:
1) Mode of action: GnRH Agonist
2...
-
Product CDMO
• Cytotoxic Injection (Liquid , Lyophilized) • ONCOLOGY • CYTOTOXIC API • EU GMP • JGMP (Japan) • KGMP (Korea) • PIC/S
-
Product Buslera (Busulfan)
BUSLERA 60mg/ 10ml Vial Containing Concentrated Solution For IV Infusion
BUSLERA belongs to “alkylating agents” group of medicines, contains busulfan as active ingredient and used to clear away present bone-morrow before bone-marrow transplations.
-
Product IV Bags
PVC/PVC-free, Single- and Multi-chamber, customised size, configuration, injection ports and connectors.
Haemopharm is qualified as a leader in the infusion sector, thanks to a very important competitive advantage, represented with the exclusive and proprietary machinery for manufacturing of... -
Product Injectables
Recipharm has extensive experience in the injectables area and supplies small volume parenterals in ampoules and vials, as well as in cartridges. State of the art leak detection and automatic inspection equipment is used and Recipharm’s full service offering is complete with secondary packaging and tempera...
-
Product Food Supplements
We develop and produce food supplements in these pharmaceutical forms:
Tablets
Syrups
Drops
Oils
Capsules
Sachets
Vials
Soft Capsules
Stick pack
-
Product Injectable vials in molded glass - Clareo
Clareo, the highest quality Type II premium injection vials Clareo, the highest quality Type II premium injection vials Clareo vials are made of Type II glass and offer a combination of homogeneous wall thickness with superior cosmetic qualities suitable for high value products. Available fro...
-
Product DOXYBIO - DOXYCYCLINE 100 MG - INJECTABLE
Doxycycline is a broad-spectrum tetracycline-class antibiotic used in the treatment of infections caused by bacteria and certain parasites. It is used to treat bacterial pneumonia, acne, chlamydia infections, Lyme disease, cholera, typhus, and syphilis. It is also used to prevent malaria in combination wit...
-
Product Bonharen - Veterinary joint desease treatment
Veterinary drug, treatment of joint disease in horses and dogs. 1 % solution of sodium hyaluronate for IVN application. High efficacy, low levels of adverse reactions

-
Product Hyalgan 20 mg/2 ml
Fidia Farmaceutici Spa offers a wide range of joint healthcare products which includes hyalgan. Contact us for more information.
-
Product Datwyler's components for Vial applications
Datwyler is the preferred solution partner to global pharmaceutical companies. We offer one of the most extensive product portfolios for vial applications in the pharmaceutical and biotech markets worldwid. Our range of products and services includes the most innovative elastomer formulations, coatings, al...
-
Product Formulation Development Services
Aurigene Pharmaceutical Services offers end-to-end formulation development services ranging from early development to clinical supplies for new chemical entities for the pharmaceutical, nutraceutical & animal healthcare industry along with life cycle extension of the products. We have the expertise alo...
-
Product Bendamustine AqVida 100 mg/ml concentrate for solution for infusion
Filling Sizes: 50 mg/ 0,5 ml; 100 mg/ 1 ml; 200 mg/ 2 ml

-
Product CDMO Best Practices from Benches to Batches - How to Transition from Clinical to Commercial Readiness
What you need to seamlessly transition from clinical phases to commercial readiness.
Three unique abilities your CDMO must have to complete your project within your timeline are capability, flexibility and availability.
August Bioservices' Director of Customer Operations, Commercial Devel...
-
Product RayDyLyo® Vial adapter
ARaymondlife has developed the essential accessory for drug reconstitution, the vial adapter, one for each RayDyLyo diameter. The RayDyLyo® vial adapter is a medical device intended for the quick and safe reconstitution of drugs, contained in vials capped with RayDyLyo® plastic press fit caps, a...
-
Product Pronolis
The first High Density (HD) Hyaluronic Acid gel: Complete and innovative range of viscosupplementation. Medical Device Class III
• High quantity of hyaluronic acid, up to 120mg per syringe • High concentration, for a fast and prolonged pain relief • Unique Mono-shot HD hyaluronic acid specificificall...
-
Product Generic (Voriconazole)
- Active substance(s): Voriconazole- Pharmaceutical form(s): Powder for solution for infusion
- Strength(s): 200 mg - IV
-
Product INVIMA Approved Oncology
Lodaat's Oncology Colombian INVIMA approved injectables with CTD dossier
-
Product Amikacin Inj.Sol 500mg/2ml (EU CTD Available) & 1g/4ml (EU CTD Available: Q3 2021)
Amikacin Solution for Injection
Reference Product: Briklin® / BRISTOL-MYERS SQUIBB
Description: Amikacin sulfate is indicated in the short-term treatment of serious infections due to susceptible strains of Gram-negative bacteria, including Pseudomonas species,&nb...
-
Product Prothrombin Complex 250IU, 500IU powder and solvent for solution for injection
Contact us for more information.
-
Product Gentamicin DS 40 mg/ml solution for injection 2 ml
Gentamicin belongs to the group of aminoglycoside antibiotics. Aminoglycosides are broad-spectrum antibiotics, particularly effective against aerobic and facultative anaerobic Gram-negative bacteria such as the family of Enterobacteriaceae and Pseudomonas aeruginosa. They exert a rapid bactericidal effect ...
-
Product Cnoxane Inj. (Enoxaparin Sodium)
• Generic name: Enoxaparin Sodium 20/40/60mg • Indication: LMWH: Anti-coagulants • First generic in Korea
-
Product Pantoprazole Sodium for Injection
Mainly used for 1. Peptic ulcer bleeding; 2. Acute gastric mucosal damage caused by non-steroidal anti-inflammatory drugs and the occurrence of ulcer hemorrhage under stress; 3. After general anesthesia or major surgery, as well as debilitating coma patients to prevent gastric acid reaction flow with aspir...
-
Product Ampicillin sodium for injection
Reyoung Pharmaceutical Co. Ltd. is one of the leading manufacturer and distributor of pharmaceutical products.
It has 31 workshops, which can produce ten categories and more than 400 specifications of pharmaceutical products including tablets, capsules, granules, st...
-
Product Primary Packaging - Gx® prefillable syringes
Prefillable glass syringes in bulk and "ready-to-fill" format, type 1 glass, filling volumes of 0.5 ml to 5.0 ml, luer cone and luer lock syringes, needle syringes, silicone-oil reduces Gx Baked-on RTF® syringes, metal-free Gx RTF® syringes, innovative syringe components, round and cut finger flange, most ...
-
Product Compound Dextran 40 Injection
Active Ingredients:Dextran 40 25g Sodium Chloride 50mg
Calcium Chloride 75mg
Sodium lactate 775mg
Potassium Chloride 1.5g
Indications:
...
-
Product Fondaparinux Injection
Fondaparinux is an anticoagulant medication chemically related to low molecular weight heparins.
Strength: 0.5ml:2.5mg; 0.4ml:0.5mg; 0.6ml:7.5mg; 0.8ml:10mg.
It is approved by US-FDA.
-
Product DAUNOCIN for inj. (Daunorubicin)
Anti-cancer.It contains Daunorubicin HCl 20 mg.Form: Lyophilized.
-
Product RELIEF® Range
Iasis Pharma S.A. provides a wide range of products which includes Relief® solution for injection (Active ingredient: Thiocolchhicoside). It belongs to musculoskeletal system category. Active ingredient: thiocolchicoside. Contact us for more information.
-
Product Vial Washing Machine
Compromec offers wide range of pharmaceutical equipments which includes vial washing machine. It belongs to liquid and lyophilised product vial filling line category. Features: it is designed with individual water and air cleaning station as cgmp standards, the machine ultrasonically cleans (option), autom...
-
Product Dual-Mix® Flexible Bags - Innovative Error-free System for Drug Reconstitution
Technoflex has developed Dual-Mix ®, an innovative primary packaging which meets three challenges: storing the medicine to be reconstituted (powder or lyophilizates) and its diluent, making its reconstitution secure and ensuring the safety of healthcare staff and the patient. • Ready-to-Use • Peel...
-
Product PHENOBARBITAL SODIUM INJECTION 100MG/ML or 200MG/ML
Phenobarbital Sodium Injection 15mg/ml, 30mg/ml, 60mg/ml, 100mg/ml or 200mg/ml- Phenobarbital belongs to a group of drugs known as barbiturates. Barbiturates have antiepileptic properties which mean they help prevent some types of epileptic seizures. They can also make you feel sleepy. Phen...
-
Product Cefadroxil Capsules
Jingxin Pharmaceutical offers a wide range of finished dosage forms products which includes cefadroxil capsules. It belongs to cephalosporin category. Contact us for more information.
-
Product Analytical services
West offers analytical services for specialized testing and analysis to evaluate the compatibility of drug products with their packaging or administration system for pharmaceutical and biopharma customers.
-
Product THERMOFORMING MACHINES FOR TRAYS SERIES "RF"
Automatic thermoforming machines to handle syringes, vials, ampoules or similar products into trays by using thermoforming material from reel.
Availability in different versions in accordance with customer's exigencies:- mechanical version- electronic version equipped with mechanical pliers - elect...
-
Product Oval Shaped PE Containers
Vioser provides a wide variety of pharmaceutical products and medical equipment which includes oval shaped PE containers. It is produced using the bottlepack system of blow-fill-seal technology. Features: Twin port closure system for infusion and additions, non fragile, easy to store, cost-effective, long ...
-
Product Fluphenazine Decanoate Injection
A clear, slight
yellowish oily liquid
filled in glass container.
-
Product Products
Please follow the following link to view our Product Catalogues:
https://www.pharmathen.com/portfolio
Contact us for more information.

-
Product Euroject®
Recent innovations have opened up the possibility of using BFS technology to design aseptic packaging systems combining the efficiency of high-throughput production with the highest guarantees of sterility. The result is a new type of pre-filled, single-dose disposable device that can be produced...
-
Product Compact Dual-Chamber Autoinjector
A REVOLUTIONARY TWIST ON EMERGENCY AUTOINJECTORS The thermally stable drug delivery platform for automatic mixing and rapid dissolution of delivered doses up to 0.3 mL.
-
Product Implanters: Solutions for sustained released formulations
Implants are fragile and insertion into the body requires caution. Nemera developed a portfolio of devices to deliver implants with integrated needlestick protection. As implants can vary a lot, we support your projects and explore design attributes that will increase the user experience such as improved h...
-
Product INJECTABLES
Requiring injectables.EU-GMP is a must.
EU dossier is a must.
Licensing and supply model or distributorship.
-
Product Dexmedetomidine ready to use, 4mcg/ml (vial)
For sedation of adult ICU (Intensive Care Unit) patients requiring a sedation level not deeper than arousal in response to verbal stimulation (corresponding to Richmond Agitation-Sedation Scale (RASS) 0 to -3).
For sedation of non-intubated adult patients prior to and/or during diagnostic or surgical ...
-
Product Liposomal DOxorubicine inj
Liposomal doxorubicin is doxorubicin contained in tiny spheres called pegylated liposomes. These spheres keep the doxorubicin in the bloodstream longer, so that more of the drug reaches the cancer cells. Indication: i) As monotherapy for patients with metastatic breast cancer, where there is an increa...
-
Product Eribulin Mesylate Injection
Generic Name: Eribulin Mesylate Injection
Indication: Metastatic breast cancer (CN, US, EU, Japan and other countries)
Liposarcoma (US, EU)
Soft tissue sarcoma (Japan and other countries)
Guidelines:
NCCN (2023V4), CSCO (2023), ESMO (2023)
Exc...
-
Product INJ.LEUCOVORIN CALCIUM 50 MG
- AN ACTIVE METABOLITE OF FOLIC ACID ( ALSO CALLED FOLINIC ACID AND CICTROVORUM FACTOR ), WHICH DOSE NOT REQUIRE METABOLISM BY DIHYDROFOLATE REDUCTASE , THE MOLECULAR TARGET OF FOLATE ANTAGONIST- TYPE CHEMOTHERAPEUTIC DRUGS.
-
Product vialose™ trehalose dihydrate
vialose™ trehalose dihydrate is a premier performance sugar that is resistant to acid hydrolysis and enzymatic cleavage. It is used to protect and shield biologic APIs, recombinant proteins, and monoclonal antibodies (mAbs) from degradation and aggregation and to stabilize and protect valuable componen...
-
Product Biologic drug substance CDMO services
From pre-clinical development to commercial supply, Patheon by Thermo Fisher Scientific is an industry leader in the development and manufacture of mammalian cell culture drug substances. Patheon offers biotech and pharmaceutical companies the ability to pursue opportunities around the globe with a fully i...
-
Product FDF Product Portfolio
Finished Dosage Forms
Beta-lactam antibiotics
Amoxicillin Amoxicillin + Clavulanic acid
Statins
Atorvastatin Rosuvastatin
Anti-fungals
Caspofungin
Sterile injectables
Semi-Synthetic Penicillins (SSP)
Amoxicillin & Cloxacillin Ampicillin Sodium Amoxicill...
-
Product Walk in Cooling Cabinet.
The Walk-In Cooling Cabinets / cold rooms are designed according to ICH guidelines, WHO, MCA and USFDA requirements to maintain uniform conditions. They are superior in airflow distribution, temperature control technology, cabinet construction and are manufactured as per cGMP regulations.
Biggest Walk...
-
Product INJECTA 36 - High-speed, advanced robotic processing for Ready-To-Use syringes
Enhancing the performance of advanced robotics, the INJECTA 36 raises the bar for high-speed production of Ready-To-Use syringes and pre-capped cartridges. The same cutting-edge robotic technologies assure accurate no-touch-transfer component processing with minimal operator access to the working area....
-
Product Acyclovir sodium sterile
Acyclovir Sodium Injection is a synthetic nucleoside analog, active against herpes viruses. It is a sterile, aqueous solution for intravenous infusion, Ayclovir Sodium Injection is a synthetic nucleoside analog, active against herpes viruses.
Acyclovir Sodium is the sodium salt form of a...
-
Product Vial Adapter Ø13 and 20 mm
The VIAL ADAPTER is a needle free system that makes the mixing or drug reconstitution easy and safe when used with a vial. It is the ideal solution for mixing lyophilized drug when provided with fixed volume diluent vial.
- A precise mixed volume q...
-
Product CEFTRIAXONE INJ + WFI 250, 500,1000
• Lower Respiratory Tract Infections .
• Acute Bacterial Otitis Media
• Skin And Skin Structure Infections
• Urinary Tract Infections (Complicated And Uncomplicated)
• Uncomplicated Gonorrhea (Cervical/Urethral And Rectal)
...
-
Product TRABECTEDIN
0.25 mg/ 1 mg powder for concentrate for solution for infusion | Therapeutic use: Advanced soft tissue sarcoma and relapsed platinum-sensitive ovarian cancer | ATC: L01CX01

-
Product Ferrous Bisglycinate
Dr. Paul Lohmann® offers a wide range of high-quality Mineral Salts suitable for remedying mineral deficiencies. These include highly soluble mineral aspartates, excellently bioavailable ferrous bisglycinate, injectable sodium dihydrogen phosphate 2-hydrate and many more. Please contact us for mor... -
Product Sterile drug products
• Injectable (freeze dried products, liquids) • Vials (aseptic filling) • Ampoules (closed, final sterilization) • Sterile powder
-
Product Drug Product Manufacturing
Ajinomoto Bio-Pharma Services is an established premier CDMO for large, medium, and small molecule APIs offering a unique range of formulation and aseptic filling in vials, prefilled syringes and cartridges to address production needs that span from clinical to commercial phase products. Our global netw...
-
Product EXCIPIENTS FOR INJECTABLE PRODUCTS
LACTEL BIODEGRADABLE POLYMER: DURECT CORPORATION USAINJECTABLE GRADE OILS (REFINED SOYABEAN OIL, REFINED OLIVE OIL, REFINED SESAME OIL): SIO-ADM FRANCE
EGG YOLK LECITHIN AND SODIUM OLEATE: DOOSAN CORPORATION, KOREA
SULFOBUTYL ETHER BETACYCLODEXTRIN (CAPTISOL): CYDEX, USAPOLY VINYL ALCOHOL: NIPPON GOH...
-
Product Nimesulide inj. 100 mg/ml
Nimesulide is a non-steroidal anti-inflammatory drug (NSAID) that has antipyretic and analgesic properties. The anti-inflammatory, analgesic and antipyretic activities of nimesulide.
-
Product DACTINOMYCIN FOR INJECTIONS
HISUN OFFERS SUPERIOR SEVICE AND TECNICAL EXPERTISE THATS POSITION US AMONG LEADING CMOS IN CHINA , FOR FURTHER INFORMATION PLS. CONTACT [email protected].
-
Product Metaraminol Bitartrate injectable vial 10mg/1ml
Metaraminol Bitartrate injectable vial 10mg/1ml. Dossier e-CTD available with 2 years stability data.
-
Product Piercan Gloves
With two upgraded manufacturing factories located in San Marcos, California and Port en Bessin, France Piercan service global markets with its quality containment gloves. Our trained staff will help provide knowledge of each glove's characteristics for chemical resistance, sterilization compatibility,...
-
Product DAYTRIX
1g/3,5ml powder and solvent for injectable solution for IM use box of 1 vial powder + 1 ampoule solvent. API: Ceftriaxone
-
Product Cloxacillin inj 1g & capsules 500 mg
Reig Jofre Group offers wide range of pharmaceutical products which includes anaclosil. Packaging: anaclosil 500 mg capsules, 500 cap. E.c, anaclosil 500 mg capsules, 20 capsules, anaclosil 500 mg capsules, 40 capsules, etc.
-
Product Acyclovir 500mg/1g injection
Acme formulation pvt.ltd. Offers a wide range of pharmaceutical products which includes Acyclovir 500mg/1g injection. It belongs to sterile lyophilized injection. Contact us for more information

-
Product Donepezil Depot Injection
Dongkook Pharm. Co. Ltd offers a wide range of finished pharmaceutical products which includes Donepezil Depot Injection. Active ingredients: 1 vial contains 0.27mg of donepezil (as a donepezil base). Indication: dementia of the Alzheimer's type. Shelf life: 2 years. Stor...
-
Product Extractable & Leechables
Our extractable and leachable services include:• Design, develop and execute the E&L study for pharmaceutical industry according to the variousregulatory guidelines and recommendations such as USFDA (USP <1663> and <1664> General Chapters),EMA, PQRI, ICH and/or customer requirements.• Selec...
-
Product Biolevox™ intraarticular injections
Biolevox™ HA 2.2% is a hyaluronic acid (HA) gel designed for a three intra-articular injection into the osteoarthritis (OA) affected in order to ameliorate symptoms of the osteoarthritis (OA) in synovial joint.Designed HA-based gel contains 44 mg of hyaluronate in 2 mL pre-filled syringe. The com...
-
Product MONOSHOT HA + PRP
Monoshot™ HA+PRP is a combination of hyaluronic acid (HA) with platelet-rich plasma (PRP), intended for intra-articular injection. Product provides simultaneous mechanical and anti-catabolic action of HA with optimally selected physicochemical parameters and the anabolic action of growth factors secre...
-
Product Progum™ HA
PROGUM™ HA is highly concentrated native hyaluronic acid (HA). The parameters of PROGUM™ HA have been carefully selected, so it can act as an initiator of regenerative processes in dental procedures. HA exhibits a range of properties beneficial for tissue regeneration, thereby supporting the healing of wou...
-
Product Lipotris
Liposomal intra-articular gel composite - new generation viscosupplement which ensures supreme lubrication mechanism. Lipotris™ therapy provides accelerated pain relief with sustainable duration of clinical effect. The TRIAD OF AN IDEAL LUBRICATION provides ultra-low friction ratio between cartilage s...
-
Product FLAVYA
Flavya™ products are injectable medical devices containing native hyaluronic acid (HA). Flavya™ complements HA deficiencies in dermis, attracts water molecules, neutralizes free radicals, creates conditions for regeneration processes and production of new collagen and elastin fibres in the skin.

-
Product Testosterone Undecanoated injection – TESTODRIOL.
Power your perfomance
Shree Venkatesh International Ltd offers a wide range of pharmaceutical products, including Testosterone Undecanoated injection – TESTODRIOL.
• Use: Testosterone Undecanoate is used in hormone replacement therapy (HRT) for men with low or absent testosterone lev...
-
Product Medroxyprogesterone Acetate Injectable Suspension USP 150mg/ml -MEDROXY 150
For Healthy Womanhood, Regular Menstrual Cycle with MEDROXY
Shree Venkatesh International Ltd offers a wide range of pharmaceutical products, including Medroxyprogesterone Acetate Injectable Suspension 150mg/ml - 1 ml Injection.
• Use: Medroxy 150 injection is used to prevent...
-
Product Norethisterone Enanthate Injection 200 mg/ ml
Long-Lasting Contraceptive Care
Shree Venkatesh International Ltd offers a wide range of pharmaceutical products, including Norethisterone Enanthate Injection 200 mg/ ml.
• Use: This injectable contraceptive is designed for long-term pregnancy prevention. • Pharmaceutical F...
-
Product Norethisterone Enanthate and Estradiol Valerate injection-Bestop
Empowering Women's Health with Long-Lasting Protection.
Shree Venkatesh International Ltd offers a wide range of pharmaceutical products, including Norethisterone Enanthate and Estradiol Valerate injection-Bestop.
• Use: his medication is used to prevent pregnancy in women...
-
Product Chorionic Gonadotrophin for Injection HCG -OVIGIL HP 10000 IU
The Gonadotropin That Delivers – Precision, Reliability & Consistency
This product is available as a combi pack of Chorionic Gonadotrophin for Injection BP 10000 IU & Sodium Chloride Injection BP 0.9% w/v (HCG Injection).
• Use: This highly purified Human Chorionic Gonadotropi...
-
Product Urofollitropin for Injection BP FSH - Ovitropin FSH 150 IU
The Gonadotropin That Delivers – Precision, Reliability & Consistency
Shree Venkatesh International Ltd offers a wide range of pharmaceutical products, including the Human Urofollitropin for Injection BP 75 IU (lyophilized, sterile powder) & Sodium Chloride Injection BP 0.9% w/...
-
Product Progesterone Injection USP 100 mg/ml-Pregaloop
Bonds the Loop Between Mother and Child
Shree Venkatesh International Ltd offers a wide range of pharmaceutical products, including Progesterone Injection USP 100 mg/ml - Pregaloop.
• Use: Progesterone Injection is used to prevent threatened abortion and preterm labor. • Pharmace...
-
Product Estradiol ( as hemihydrate) & Norethisterone Acetate Tablets - Noret
Empowering health through innovation and expertise.
Shree Venkatesh International Ltd offers a wide range of pharmaceutical products, including Estradiol ( as hemihydrate) & Norethisterone Acetate Tablets - Noret –
• Use: Hormonal therapy for effective management of ...
-
Product Caboprost Tromethamine injection USP 125 mcg/ml - Femmeprost
Each drop is important, stop bleeding after childbirth with femmeprost
Shree Venkatesh International Ltd offers a wide range of pharmaceutical products, including Caboprost Tromethamine injection USP 125 mcg/ml - Femmeprost
• Use: For the prevention and treatment of post...
-
Product FSH/Urofollitropin for Injection BP 75 IU - Ovitropin FSH 75 IU
The Gonadotropin That Delivers – Precision, Reliability & Consistency
Shree Venkatesh International Ltd offers a wide range of pharmaceutical products, including the Human Urofollitropin for Injection BP 75 IU (lyophilized, sterile powder) & Sodium Chloride Injection BP 0.9% w/v (...
-
Product CDMO: Contract Development
EVER is a leader in pharmaceutical development
Our end-to-end service covers every aspect of pharmaceutical development, from API selection to final process validation and quality assurance, with the highest level of expertise.
With ‘state of the art’ labs in Unterach ...
-
Product EVER Pharma: Product Portfolio
Our products include: • Atosiban • Cabazitaxel • Cerbrium • Cerebrolysin • Dacepton/Apomorphine • Dexemedetomidine • Docetaxel • Fulvestrant • MemoProve • Oxytocin • Paclitaxel • Pemetrexed • Tachyben • Terlipressin • Trabectedin • Eribulin
-
Product HMG/Menotropin for Injection -Humenotropin HP 150 IU
The Gonadotropin That Delivers – Precision, Reliability & Consistency
Shree Venkatesh International Ltd offers a wide range of pharmaceutical products, including the Human Menopausal Gonadotrophin 150 IU (lyophilized, sterile powder) & Sodium Chloride Injection BP 0.9% w/v (HMG Injection...
-
Product Carboprost Tromethamine Injection USP 125 mcg/1ml - FEMMEPROST
Each Drop is important, stop bleeding after childbirth with carboprost
Shree Venkatesh International Ltd offers a wide range of pharmaceutical products, including Carboprost Tromethamine Injection USP 125 mcg/1ml - FEMMEPROST
• Use: Used to treat postpartum uterine hemorrhage due to ...
-
Product Hydroxyprogesterone Caproate Inj. 500 mg/2ml
Be on Time, Reduce the Risk of Preterm Birth
Shree Venkatesh International Ltd offers a wide range of pharmaceutical products, including Hydroxyprogesterone Caproate Inj. 500 mg/ml
• Use: A progestin indicated to reduce the risk of preterm birth in women with a singleton pregna...
-
Product Progesterone Injection USP 50 mg/ml-PREGALOOP
Bonds the Loop Between Mother and Child
Shree Venkatesh International Ltd offers a wide range of pharmaceutical products, including Progesterone Injection USP 100 mg/ml - Pregaloop.
• Use: Progesterone Injection is used to prevent threatened abortion and preterm labor. • Pharmaceutical...
-
Product Human Growth Hormone rDNA Origin (HGH) Somatropin for injection USP -Shreetropin
A human growth hormone drug for dwarf child
Shree Venkatesh International Ltd offers a wide range of pharmaceutical products, including the Human Growth Hormone rDNA Origin (HGH) Somatropin for injection USP -Shreetropin.
• Use: Treatment for children with growth failure due to ...
-
Product Testosterone Compound Injection 250 mg/ml -Restanon 250
Power Your Performance
Shree Venkatesh International Ltd offers a wide range of pharmaceutical products, including Testosterone Compound Injection 250 mg/ml -Restanon 250
• Use: Increases testosterone levels in adult men, addressing issues such as impotence, infertility, low sex dri...
-
Product Somatropin for injection USP 16 IU – Shreetropin
A human growth hormone drug for dwarf child
Shree Venkatesh International Ltd offers a wide range of pharmaceutical products, including the Somatropin for injection USP 16 IU-Somatropin.
• Use: Treatment for children with growth failure due to inadequate secretion of endogenous growth...
-
Product HCG/Chorionic Gonadotrophin for Injection - OVIGIL HP 1000 IU
The Gonadotropin That Delivers – Precision, Reliability & Consistency
This product is available as a combi pack of Chorionic Gonadotrophin for Injection BP 1000 IU & Sodium Chloride Injection BP 0.9% w/v (HCG Injection).
• Use: This highly purified Human Chorionic Gonadotropin...
-
Product Oxytocin injection BP - LOVTIN
Fasten the Delivery, Slow the Pain of Labor
Shree Venkatesh International Ltd offers a wide range of pharmaceutical products, including Oxytocin Injection BP - LOVTIN.
• Use: A synthetic hormone that helps speed up delivery or controls bleeding after childbirth. • Pharmaceutical For...
-
Product Buserelin injection - FERTILIN
Buserelin: Busting Cancer at Its Core
Shree Venkatesh International Ltd. offers a wide range of pharmaceutical products, including Buserelin Injection.
• Uses: - • Primarily used in the treatment of prostate cancer and endometriosis • Also effective in treatin...
-
Product Progesterone injection 25 mg/ml - Pregaloop AQ-25
Prepare for a Healthy Pregnancy with Pregaloop AQ
Shree Venkatesh International Ltd. offers a wide range of pharmaceutical products, including Progesterone 25mg/50mg Aqua Base Injection.
• Uses: • A female hormone used in hormone replacement therapy for progestin ...
-
Product Triptorelin Acetate for Injection 3.75 mg
Advanced Care with Triptorelin Acetate Injection
Shree Venkatesh International Ltd. offers a wide range of pharmaceutical products, including Triptorelin Acetate for Injection 3.75 mg.
• Uses: • To tret advanced prostate cancer Pharmaceutical Form: Injectable • P...
-
Product Follicle Stimulating Hormone Injection BP 900 IU/1.5 ml - Goterui
Empowering Reproductive Health
Shree Venkatesh International Ltd. offers a wide range of pharmaceutical products, including Goterui — Follicle Stimulating Hormone Injection BP 900 IU/1.5 ml.
• Uses: Used to treat infertility in women, Goterui contains a man-made version of...
-
Product Human Menopausal Gonadotrophin For Injection 75 IU.
The Gonadotropin That Delivers – Precision, Reliability & Consistency
Shree Venkatesh International Ltd. offers a wide range of pharmaceutical products, including Human Menopausal Gonadotrophin For Injection 75 IU.
• Use: Specify the primary use here, e.g., "Used in fertili...
-
Product Human Menopausal Gonadotrophin For Injection 150 IU.
The Gonadotropin That Delivers – Precision, Reliability & Consistency
Shree Venkatesh International Ltd. offers a wide range of pharmaceutical products, including Human Menopausal Gonadotrophin For Injection 150 IU.
• Use: Specify the primary use here, e.g., "Used in fer...
-
Product Follicle Stimulating Hormone BP Injection 150 IU.
The Gonadotropin That Delivers – Precision, Reliability & Consistency
Shree Venkatesh International Ltd. offers a wide range of pharmaceutical products, including Follicle Stimulating Hormone BP Injection 150 IU.
• Use: This combination is used in the treatment of infert...
-
Product Follicle Stimulating Hormone BP Injection 75 IU.
The Gonadotropin That Delivers – Precision, Reliability & Consistency
Shree Venkatesh International Ltd. offers a wide range of pharmaceutical products, including Follicle Stimulating Hormone BP Injection 75 IU.
• Use: This combination is used in the treatment of inferti...
-
Product Progesterone injection 50 mg/ml - Pregaloop AQ-50
Prepare for a Healthy Pregnancy with Pregaloop AQ
Shree Venkatesh International Ltd. offers a wide range of pharmaceutical products, including Progesterone 50mg Aqua Base Injection.
• Uses: • A female hormone used in hormone replacement therapy for progestin • Helps treat...
-
Product CONDROTIDE - (POLYNUCLEOTIDES HPT™)
CONDROTIDE acts as“SLOW-ACTING “ and “DISEASE MODIFYING-PRODUCT” for OA with a clinically proven effects on:
. Pain reduction. cut of nsaids consumption
.enhancement of joint mobiity
.resumption of sporting activity
Condrotide must be administered by intra-articular injection into ...
-
Product Ondavell (Ondansteron Hydrochloride)
Ondavell contains Ondansetron, which belongs to antiemetics category. It used for preventing nausea and vomiting caused by chemotherapy (in adults and children) or radiotherapy for cancer (adults only) and after surgery.
Available in film-coated tablet, orally disintegrating tablet (4 mg a...
-
Product Xevolac (Ketorolac Trometamol)
Xevolac contains ketorolac trometamol, a "Non-Steroidal Anti Inflammatory Drug or NSAID". it is used for pain relief after operations and could lessen pain, swelling, redness and heat (inflammation).
Available in ampoule (volume 1 mL, strength 10 mg/mL and 30 mg/mL), pre-filled syringe (volu...
-
Product Regivell Spinal (Bupivacaine Heavy) 0.5% Heavy Solution for Injection
Regivell contains bupivacaine hydrochloride which belongs to group medicines local anaesthetics. It used to numb (anaesthetise) parts of the body during surgery in adults and children of all ages, it stops pain happening during surgery: urological or lower limbs surgery, including hip surgery, and lo...
-
Product Carbetocin injection
² It is used as the first-line medication to prevent postpartum hemorrhage caused by vaginal delivery and cesarean section.
² Traditional oxytocin products have been upgraded to have stronger metabolic stability, higher bioavailability, longer acting effect, stronger potency, and are s...
-
Product ONCOLOGY LYOPHILIZED INJECTABLES
AZACITIDINE | BENDAMUSTINE | BLEOMYCIN | BORTEZOMIB | CYTARABINE | DACARBAZINE | EPIRUBICIN | GEMCITABINE | PEMETREXED | TOPOTECAN | ZOLENDRONIC ACID

-
Product ONCOLOGY LIQUID INJECTABLES
CARBOPLATIN | CISPLATIN | CYTARABINE LIQUID | DOCETAXEL WITH SOLVENT | DOCETAXEL (CTD) SINGLE VIAL | DOXORUBICIN | EPIRUBICINE | ETOPOSIDE | IRINOTECAN | LEUCOVORIN CALCIUM | METHOTREXATE | OXALIPLATIN | PLERIXAFOR | PACLITAXEL | VINCRISTINE | 5-FLOUROURACIL | FULVESTRANT

-
Product OTHER INJECTABLES
REMDESIVIR FOR INJECTION | ACECLOFENAC | CISATRACURIUM BESYLATE | CITICOLINE | DEXMETOMIDINE | L-ORNITHINE L-ASPARTATE | MIDAZOLAM INJ | N-ACETYLCYSTEINE | SUGAMMADEX| PARACETAMOL
-
Product Molly® Modular Platform Autoinjector
Molly is a single-dose autoinjector technology developed to help pharma and biotech companies reduce initial investments and expedite development timelines. Built on a proven modular platform, Molly reduces risks during the development process while increasing flexibility in device design and production. A...
-
Product Doxorubicin Hydrochloride Liposome Injection
Indications:
Ovarian Cancer
Doxorubicin Hydrochloride Liposome Injection is indicated for the treatment of patients with ovarian cancer whose disease has progressed or recurred after platinum-based chemotherapy.
Multiple Myeloma
...
-
Product NEWEST - (POLYNUCLEOTIDES HPT™ + HYALURONIC ACID)
NEWEST Suggested for application on mature and dehydrated skin.
Promotes re-modelling of striae distensae, depressed scars, and skin trophism by improving its appearance. Polynucleotides with High Purification Technology (PN HPT™) are natural origin products obtained through a technology feat...
-
Product AESTHETIC MEDICINE LINE (POLYNUCLEOTIDES HPT™/ POLYNUCLEOTIDES-HPT™ + HYALURONIC ACID)
PLINEST: Suggested for prevention and maintaining younger patient’s skin quality. It helps to remodel areas with a high fibrous content such as acne scars
PLINEST EYE: Suggested for improving periocular skin texture
PLINEST HAIR: Su...
-
Product PLINEST - (POLYNUCLEOTIDES HPT™)
PN HPT™ are deoxyribonucleotide polymers (DNA fractions) with a crucial role in: • Skin quality improvement • Restoring radiance and freshness • Promoting bio-regeneration • Reducing wrinkles and skin laxity
The Polynucleotides with HPT™ PRIMING process prepare the skin and ...
-
Product Playal®
INJECTIONS Patented formulation featured by Sodium Hyaluronate at high molecular weight and Niacinamide available in different concentrations and volume.
Playal® 20 mg/2mL;
Playal® 30 mg/2mL;
Playal® 36 mg/2mL;
Playal® 40 mg/2mL;
Playal® 80 mg/4 mL. q...
-
Product Promovia®
INJECTIONS Family of pre-filled syringes of Sodium Hyaluronate at medium molecular weight available in different concentrations and volumes.
The brand, depending on the territory, can be commercialized under the brand name of Viscoial.
Promovia® 2 (24 mg/2mL);
Promovia® 2 (4...
-
Product GMP Analytical Testing
Singota solutions offers wide range of services which includes GMP analytical testing.
Studies & Methodologies: • Material Identity • In Process • Product Release • Product Specific Assays • Compendial • Microbial (Endotoxin, Bioburden, Sterility) • CoA Generation • ICH Stability (All ICH...
-
Product Aseptic Filling
We specialize in producing small volume batches with a focus on tailored solutions, minimized line loss, and reduced lead times.
Singota's robotic aseptic filling service is supported by utilizing the latest technology - a Cytiva (formerly Vanrx) SA25 robotic workcell, featuring a complete...
-
Product Dexmedetomidine EVER Pharma
Dexmedetomidine is a highly selective alpha-2 adrenergic receptor notable for its ability to provide sedation without the risk of respiratory depression.
The first Dexmedetomidine in Europe indicated for both ICU and procedural sedation.
-
Product Glass Vials
From 1 ml to 30 ml glass vials. Type I, II and III glass, depending on your needs Amber and clear colours Normal, siliconized or ready for lyophilization Prewashed, in RTU packaging and nesting All in ISO standard measurements Capacity to develop according to client's specifications
-
Product Pharmaceutical Vials: Labelling and Packaging
Our team of specialists can tailor our offering to manage the most complex packaging requirements. Our capabilities include: - Vial labelling, re-labelling and over-labelling - Cartoning of single and multi-packs - Cold chain packaging and storage - Kitting https://youtu.be/hzKBghVqFTc
-
Product Pre-filled Syringes: Assembly, Labelling and Packaging
At Tjoapack, we provide expert packaging solutions for Pre-Filled Syringes, including assembly, labelling, blistering and cartoning. Our line is able to pack Pre-filled syringes of 1 to 5 ml, with or without safety device, and has the opportunity to manually add customer specific components. Our expertise...
Injectable Pharmaceuticals
Injectable pharmaceuticals are drug product delivery systems that can be used as an alternative to oral drugs and are more effective because of how fast they work. Injectable pharmaceuticals are passed into the human body through syringes to the bloodstream and other parts of the body. Unlike oral drugs, injectable drugs do not get dismantled in the stomach and are not altered by digestive enzymes. There are several benefits of injectable pharmaceuticals, and that is why many biotech companies and big pharmaceuticals are keying into the many opportunities in this industry.
What are the top companies that manufacture injectable pharmaceuticals?
There are many existing companies and vendors and a lot more coming up with new syringe technology ideas. Quality, financial capacity, knowledge, and experience of the industry are all key parameters in which to judge an injectable manufacturing company.
It is almost impossible to make mention of injectable pharmaceuticals without referencing Pfizer. In 2015, the company completed its buyout of Hospira, a biosimilar company and one of the top suppliers of generic injectable drugs such as anesthesia, oncology, cardiovascular, and anti-ineffective therapies.
Other big names are Hikma, Sagent and Novartis.
What are the main challenges of manufacturing injectable pharmaceuticals?
While many patients find injectable pharmaceuticals more effective than oral medicine, injectable drugs are less convenient for patients, raising issues of adherence, even for those that are in chronic conditions.
The quest for different delivery methods is another challenge even though it is being managed with the recent growth of the global injectable drug delivery market. Patients, regardless of their state, want convenience and as a result, manufacturers are faced with the challenge of meeting this demand while making sure that the right dosage form is provided.
Lack of finances can also be a significant challenge to manufacturing injectable pharmaceuticals.
What factors are driving trends in injectable pharmaceuticals?
The primary factor that appears to be driving trends in injectable pharmaceuticals is the increasing prevalence of chronic diseases. With more and more people falling ill with chronic conditions, demand for injectable drugs has increased.
Another key factor is the advancement in technology, which has contributed in no small way to patient compliance with injectable drugs.
Implementing innovations in injectable drug delivery
The biotech and pharmaceutical companies need to align their drug development with the several diseases and viruses that continue to spring up. Drug manufacturers worldwide invest a lot of money into this because it is a very long process that requires attention to details through the development, clinical trials, injection molding and approval stages. The clinical trial stage is by far the most complex of these stages because the drug has to be tested and properly evaluated to be sure it is efficient and safe for use by a large number of people.
What are the benefits of injectable pharmaceuticals?
Many people prefer to take injections than to swallow several pills, because the former are usually faster in action. Dosage of injectable drugs can be controlled: One of the significant challenges that are being faced by drug industries is drug abuse, where many use more than the prescribed amount of drug. Injectable pharmaceuticals can control this usage as professionals who are at the developing end of these pharmaceutical devices can control the quantity and concentration.
What are the limitations of injectable drug delivery devices?
The injectable drug delivery and injection molding industry is a highly innovative one, and that is why we have seen the development of many devices over the years. However, the sector still has limitations.
Recently, the injection molding industry has considered a move towards more customized hand-held devices that take advantage of existing platforms. The challenge, however, with this is the difficulty that comes with customizing the platforms. To successfully make use of these platforms, biotech and pharmaceutical companies need to differentiate them from existing ones to reach their target market faster.
Another major limitation of injectable drug delivery devices is the need for human factor engineering in designing hand-held devices. Getting the required skill on a larger scale has not been possible and has largely affected the industry projections with these devices' design. Human factor engineering is crucial for developing any connected drug delivery device in today's world. The human factor focuses on the essential elements of human interactions.
Limitation also exist in the form of regulatory guidance issued by government authorities. While the essence of these guidelines is to ensure there is a uniform framework that explains how human factor engineering and studies should be integrated into drug delivery devices, the process of seeking approval is lengthy and appears almost impossible for everyone other than the big biotech companies. This means that the availability of injectable drug delivery devices is only limited to big biotech and pharmaceutical companies' capacity.
What are some recent advances in injectable drug delivery technology?
Efforts are being made to design drug delivery devices and implement client needs into such design. An example of this is the drug delivery device of Duoject that not only focuses on meeting the mechanical needs, but has also developed a design methodology that ensures its devices are comprehensive, meeting different end-users' needs.
A notable delivery device of Duoject is its Vaccject – a cartridge-based device that serves as an alternative to the popular prefilled syringe. This delivery device comes with an auto-retract needle safety feature that can be separated from the device until you want to use it.
Another recent advancement that we have seen with injectable drug delivery technology is Enable injector development, which comes in a smaller and lighter size. It has a long-term container closure that is not contained in the device, so it is not as heavy as other devices. It also comes with a 'pause' function that users can make use of at any time and makes it possible for users to stop injecting the drug when they experience any pain or discomfort in the process.
Many biotech companies are working to deliver smarter and more effective auto-injectors. A significant consideration for the companies that produce such devices is the ability to customize an auto-injector to fit a particular company's portfolio.
Injectable pharmaceutical processing and packaging
After successfully manufacturing injectable pharmaceuticals, the next step is to have them processed and packaged. Over the years, we have seen several changes in how drugs are generally being packaged. Injectable drugs have not been different as they have come in various delivery systems that have changed over the years.
The most important elements of injectable pharmaceutical processing and packaging include the need for quality, good administration systems, and the best components. There is also the need to be aware of the regulatory guidelines that mitigate risk during packaging. Worthy of mention is the need for advanced technologies as this makes processing and packaging faster and more effective.
What materials are commonly used in injectable primary pharmaceutical packaging?
The most common primary materials used in injectable pharmaceutical packaging are prefillable glass syringes and vials for glass packaging. While these are the major materials for modern aseptic injectables, others are still being used. Some manufacturers use various glass container systems that help them customize the appearance of the injectable drug. These glass containers are used mainly for storing parenterals. Other materials needed in the packaging process include a container closure system, reconstitution kits, disposable syringes, ampoules, and pen systems.
Depending on the manufacturer and the type of design they look to achieve like plastic injection molding, they can also consider using other materials for drug delivery devices. However, it is imperative to consider end-users' needs when packaging these injectable drugs, especially because of the low compliance level of patients.
Injectable pharmaceuticals are drug product delivery systems that can be used as an alternative to oral drugs and are more effective because of how fast they work. Injectable pharmaceuticals are passed into the human body through syringes to the bloodstream and other parts of the body. Unlike oral drugs, injectable drugs do not get dismantled in the stomach and are not altered by digestive enzymes. There are several benefits of injectable pharmaceuticals, and that is why many biotech companies and big pharmaceuticals are keying into the many opportunities in this industry.
What are the top companies that manufacture injectable pharmaceuticals?
There are many existing companies and vendors and a lot more coming up with new syringe technology ideas. Quality, financial capacity, knowledge, and experience of the industry are all key parameters in which to judge an injectable manufacturing company.
It is almost impossible to make mention of injectable pharmaceuticals without referencing Pfizer. In 2015, the company completed its buyout of Hospira, a biosimilar company and one of the top suppliers of generic injectable drugs such as anesthesia, oncology, cardiovascular, and anti-ineffective therapies.
Other big names are Hikma, Sagent and Novartis.
What are the main challenges of manufacturing injectable pharmaceuticals?
While many patients find injectable pharmaceuticals more effective than oral medicine, injectable drugs are less convenient for patients, raising issues of adherence, even for those that are in chronic conditions.
The quest for different delivery methods is another challenge even though it is being managed with the recent growth of the global injectable drug delivery market. Patients, regardless of their state, want convenience and as a result, manufacturers are faced with the challenge of meeting this demand while making sure that the right dosage form is provided.
Lack of finances can also be a significant challenge to manufacturing injectable pharmaceuticals.
What factors are driving trends in injectable pharmaceuticals?
The primary factor that appears to be driving trends in injectable pharmaceuticals is the increasing prevalence of chronic diseases. With more and more people falling ill with chronic conditions, demand for injectable drugs has increased.
Another key factor is the advancement in technology, which has contributed in no small way to patient compliance with injectable drugs.
Implementing innovations in injectable drug delivery
The biotech and pharmaceutical companies need to align their drug development with the several diseases and viruses that continue to spring up. Drug manufacturers worldwide invest a lot of money into this because it is a very long process that requires attention to details through the development, clinical trials, injection molding and approval stages. The clinical trial stage is by far the most complex of these stages because the drug has to be tested and properly evaluated to be sure it is efficient and safe for use by a large number of people.
What are the benefits of injectable pharmaceuticals?
Many people prefer to take injections than to swallow several pills, because the former are usually faster in action. Dosage of injectable drugs can be controlled: One of the significant challenges that are being faced by drug industries is drug abuse, where many use more than the prescribed amount of drug. Injectable pharmaceuticals can control this usage as professionals who are at the developing end of these pharmaceutical devices can control the quantity and concentration.
What are the limitations of injectable drug delivery devices?
The injectable drug delivery and injection molding industry is a highly innovative one, and that is why we have seen the development of many devices over the years. However, the sector still has limitations.
Recently, the injection molding industry has considered a move towards more customized hand-held devices that take advantage of existing platforms. The challenge, however, with this is the difficulty that comes with customizing the platforms. To successfully make use of these platforms, biotech and pharmaceutical companies need to differentiate them from existing ones to reach their target market faster.
Another major limitation of injectable drug delivery devices is the need for human factor engineering in designing hand-held devices. Getting the required skill on a larger scale has not been possible and has largely affected the industry projections with these devices' design. Human factor engineering is crucial for developing any connected drug delivery device in today's world. The human factor focuses on the essential elements of human interactions.
Limitation also exist in the form of regulatory guidance issued by government authorities. While the essence of these guidelines is to ensure there is a uniform framework that explains how human factor engineering and studies should be integrated into drug delivery devices, the process of seeking approval is lengthy and appears almost impossible for everyone other than the big biotech companies. This means that the availability of injectable drug delivery devices is only limited to big biotech and pharmaceutical companies' capacity.
What are some recent advances in injectable drug delivery technology?
Efforts are being made to design drug delivery devices and implement client needs into such design. An example of this is the drug delivery device of Duoject that not only focuses on meeting the mechanical needs, but has also developed a design methodology that ensures its devices are comprehensive, meeting different end-users' needs.
A notable delivery device of Duoject is its Vaccject – a cartridge-based device that serves as an alternative to the popular prefilled syringe. This delivery device comes with an auto-retract needle safety feature that can be separated from the device until you want to use it.
Another recent advancement that we have seen with injectable drug delivery technology is Enable injector development, which comes in a smaller and lighter size. It has a long-term container closure that is not contained in the device, so it is not as heavy as other devices. It also comes with a 'pause' function that users can make use of at any time and makes it possible for users to stop injecting the drug when they experience any pain or discomfort in the process.
Many biotech companies are working to deliver smarter and more effective auto-injectors. A significant consideration for the companies that produce such devices is the ability to customize an auto-injector to fit a particular company's portfolio.
Injectable pharmaceutical processing and packaging
After successfully manufacturing injectable pharmaceuticals, the next step is to have them processed and packaged. Over the years, we have seen several changes in how drugs are generally being packaged. Injectable drugs have not been different as they have come in various delivery systems that have changed over the years.
The most important elements of injectable pharmaceutical processing and packaging include the need for quality, good administration systems, and the best components. There is also the need to be aware of the regulatory guidelines that mitigate risk during packaging. Worthy of mention is the need for advanced technologies as this makes processing and packaging faster and more effective.
What materials are commonly used in injectable primary pharmaceutical packaging?
The most common primary materials used in injectable pharmaceutical packaging are prefillable glass syringes and vials for glass packaging. While these are the major materials for modern aseptic injectables, others are still being used. Some manufacturers use various glass container systems that help them customize the appearance of the injectable drug. These glass containers are used mainly for storing parenterals. Other materials needed in the packaging process include a container closure system, reconstitution kits, disposable syringes, ampoules, and pen systems.
Depending on the manufacturer and the type of design they look to achieve like plastic injection molding, they can also consider using other materials for drug delivery devices. However, it is imperative to consider end-users' needs when packaging these injectable drugs, especially because of the low compliance level of patients.
References
Upcoming Events
-
CPHI Middle East 2024
Riyadh Front Exhibition & Convention Center Riyadh, Saudi Arabia
10 Dec 2024 - 12 Dec 2024 -
Pharmapack Europe 2025
Paris Expo, Porte de Versailles - Hall 7.2 | Paris, France
22 Jan 2025 - 23 Jan 2025 -
CPHI Japan 2025
East Halls 4, 5 & 6, Tokyo Big Sight, Tokyo, Japan
09 Apr 2025 – 11 Apr 2025
Pharmaceutical Industry Webinars
-
Webinar Leveraging Real-Time Clinical Data to Deliver Certainty in Solubility Enhancement and Modified Release Development
-
12th December 2024
-
3pm BST/ 4pm CET
-
-
Webinar The CPHI Sustainability Collective: A New Initiative to Support a Sustainable Pharma Value Chain
-
10th December 2024
-
3pm BST /4pm CET
-
-
Webinar Key to Success: A CDMO's Pathway to Biologics Excellence
-
5th November 2024
-
3pm BST/ 4pm CET
-
-
Webinar The Changing Dynamics of Global API Manufacturing
-
17th September 2024
-
3pm BST/ 4pm CET
-
-
Webinar Shaping the Future of Italy’s Pharma Market: Trends and Opportunities
-
4th September 2024
-
4pm CET/ 10am EST
-
-
Webinar Fragment-Based Oligonucleotide and Oligopeptide Synthesis
-
30th Jul 2023
-
4pm CET / 10am EST
-
-
Webinar GMP Rationale for Sterile High-Potency/Toxic Pharmaceuticals
-
18th June 2024
-
4pm CET / 10am EST
-
-
Webinar Unlocking Opportunities in the Growing Pharma Landscape of The Middle East
-
5th June 2024
-
3pm CET / 9am EST
-
-
Webinar Exploring Technological Trends in the Future of Pharmaceutical Manufacturing
-
23rd May 2024
-
4pm CET / 10am EST
-
-
Webinar Achieving Manufacturing Excellence Through Digital Transformation
-
16th April 2024
-
4pm CET / 10am EST
-
-
Webinar Made in Africa: What’s Driving Pharma Manufacturing
-
28th March 2024
-
4pm CET / 10am EST
-
-
Webinar Case Study: Risk Management for Annex 1 Sterile Production EMS
-
28th February 2024
-
4pm CET / 10am EST
-
-
Webinar Innovative Strategies for B2B Pharma Marketeers: Driving Value through Content
-
20th February 2024
-
4pm CET / 10am EST
-
-
Webinar Revolutionizing Pharma: Data and AI Unleashed
-
18th January 2024
-
4pm CET / 10am EST
-
-
Webinar Optimal Temperature: Elevating Biologics Cold Chain Excellence
-
16th January 2024
-
4pm CET / 10am EST
-
-
Webinar Market Outlook – The Biggest Pharma Trends of 2024
-
12th December 2023
-
4pm CET / 10am EST
-
-
Webinar The Next Frontier – Emerging Opportunities in the LATAM Pharma Market
-
21st November 2023
-
4pm CET / 10am EST
-
-
Webinar Vistamaxx™ MED - imagine the possibilities for healthcare product performance
-
10th October, 2023
-
4pm CET / 10am EST
-
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance






















































































































































































.png)
.png)
.png)
.png)
.png)
.png)
.png)

.png)





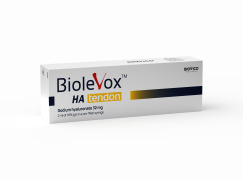


























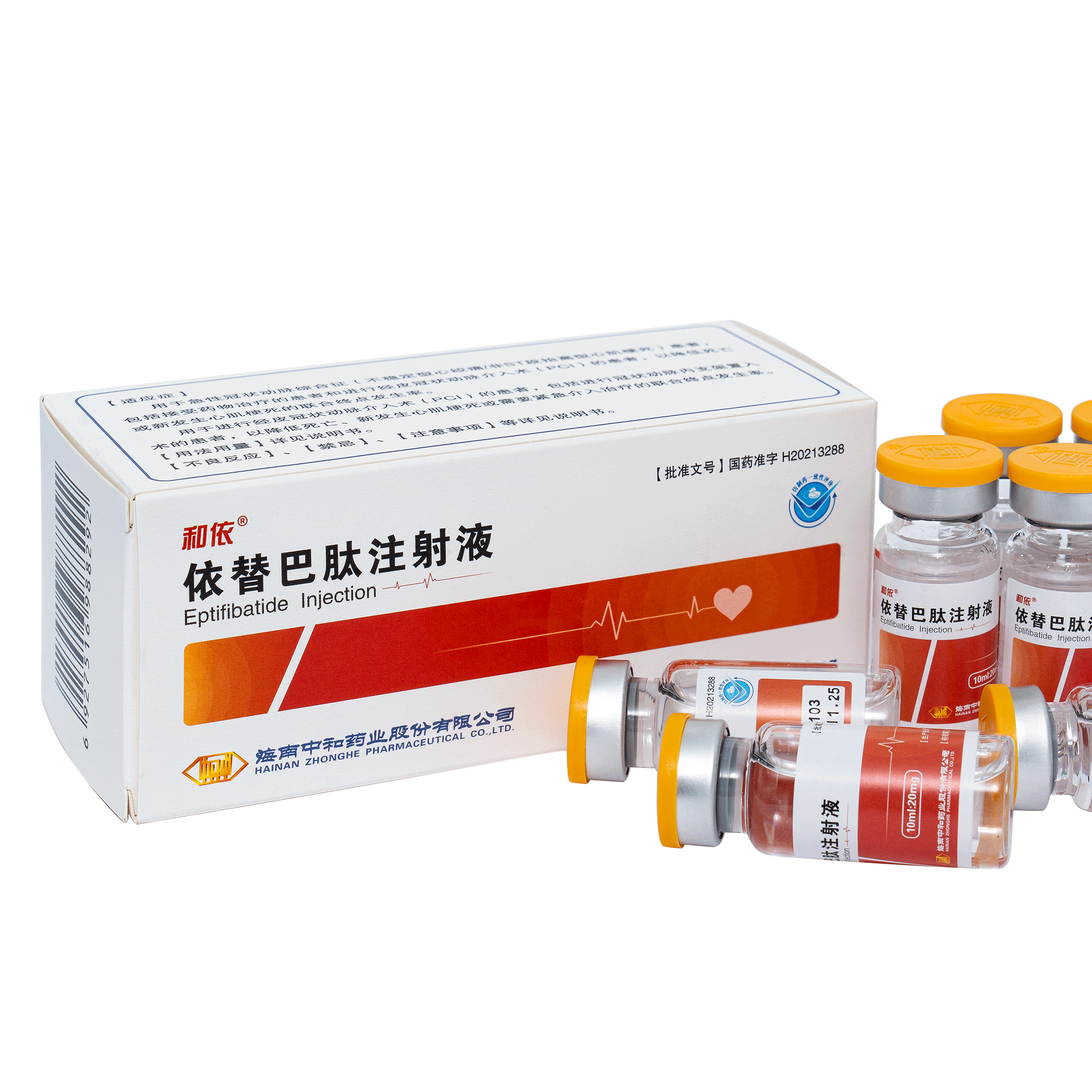





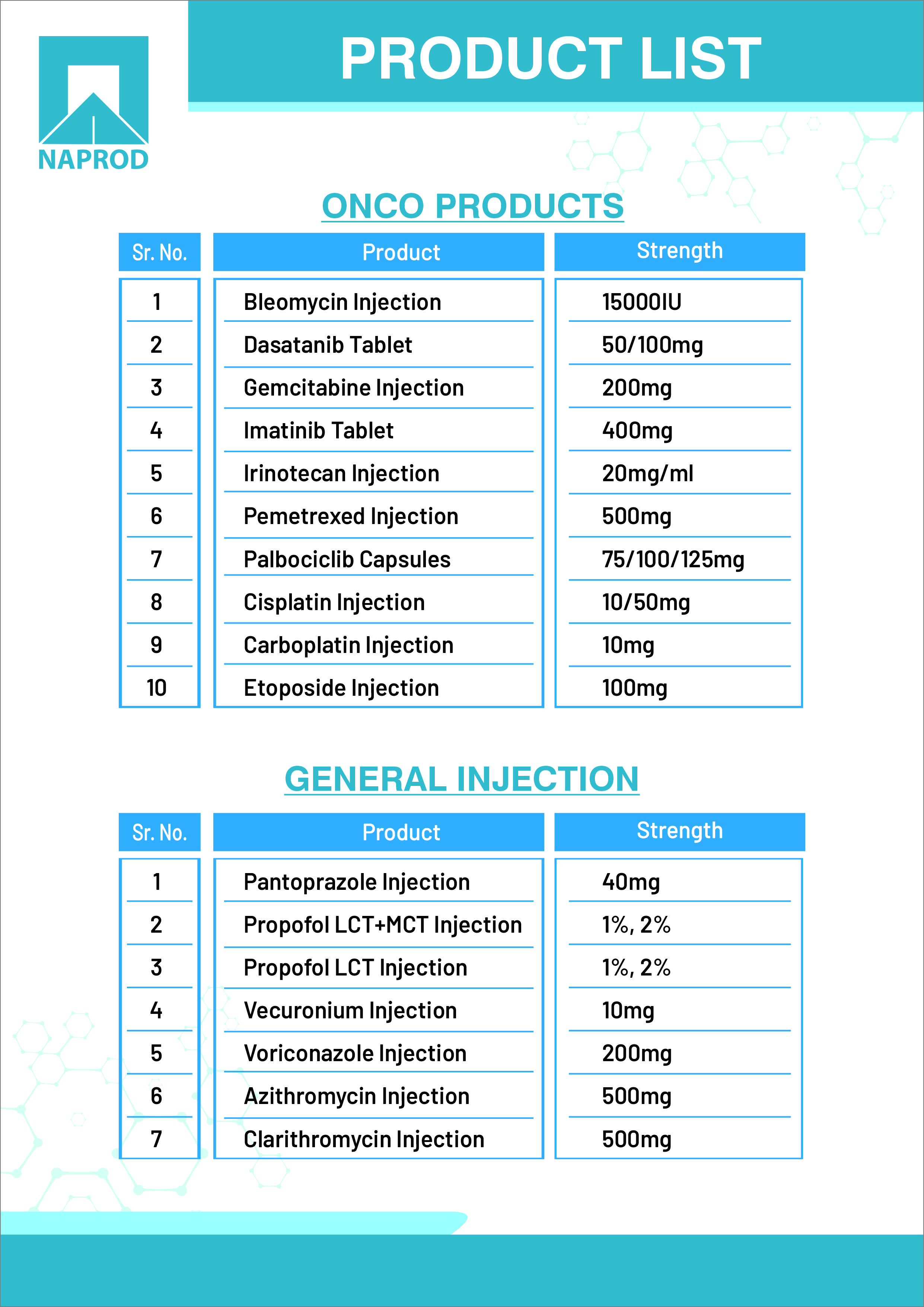










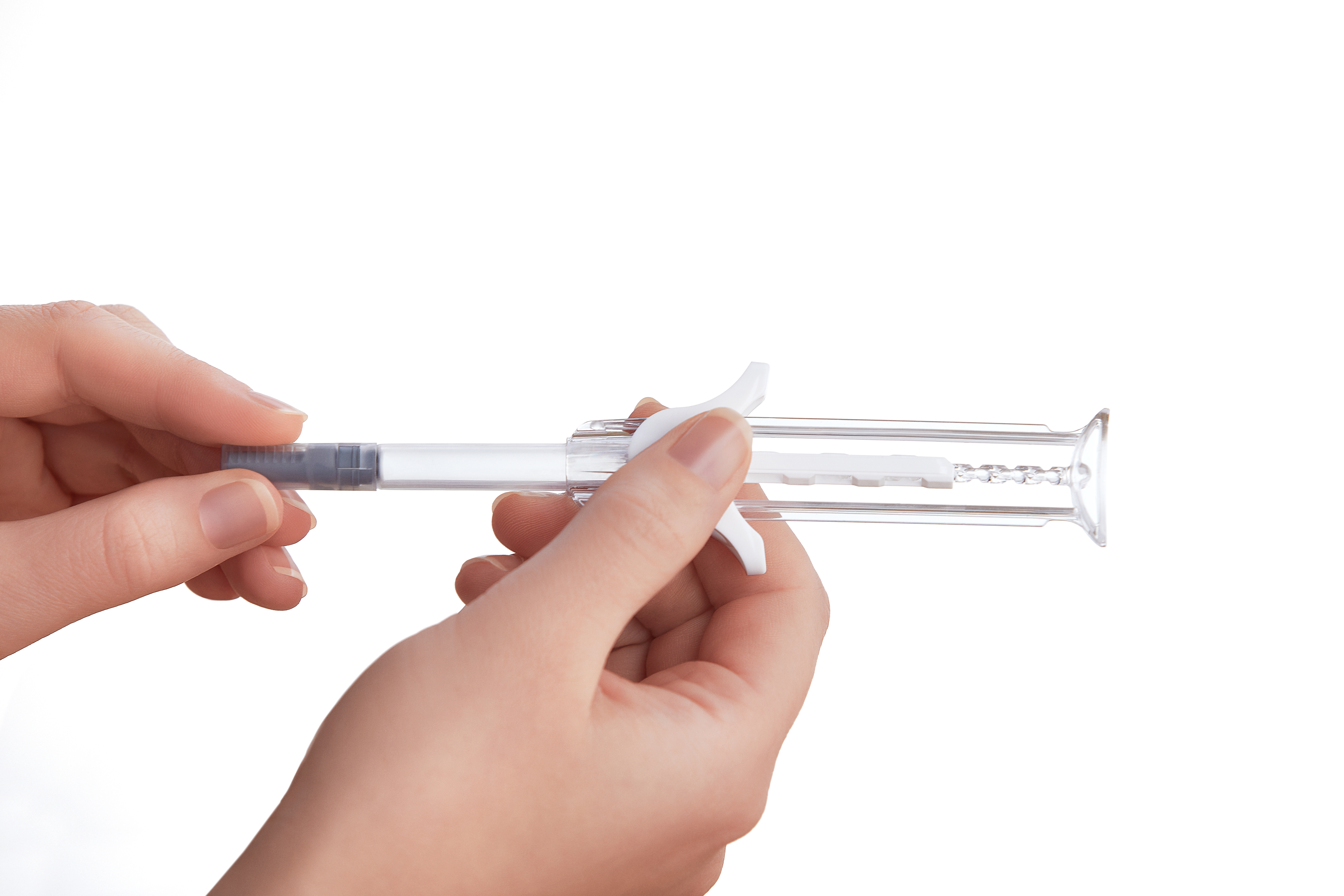
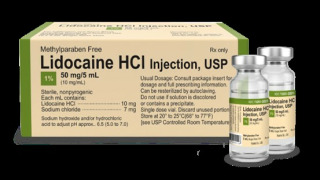






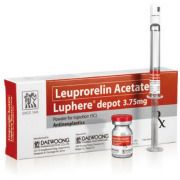



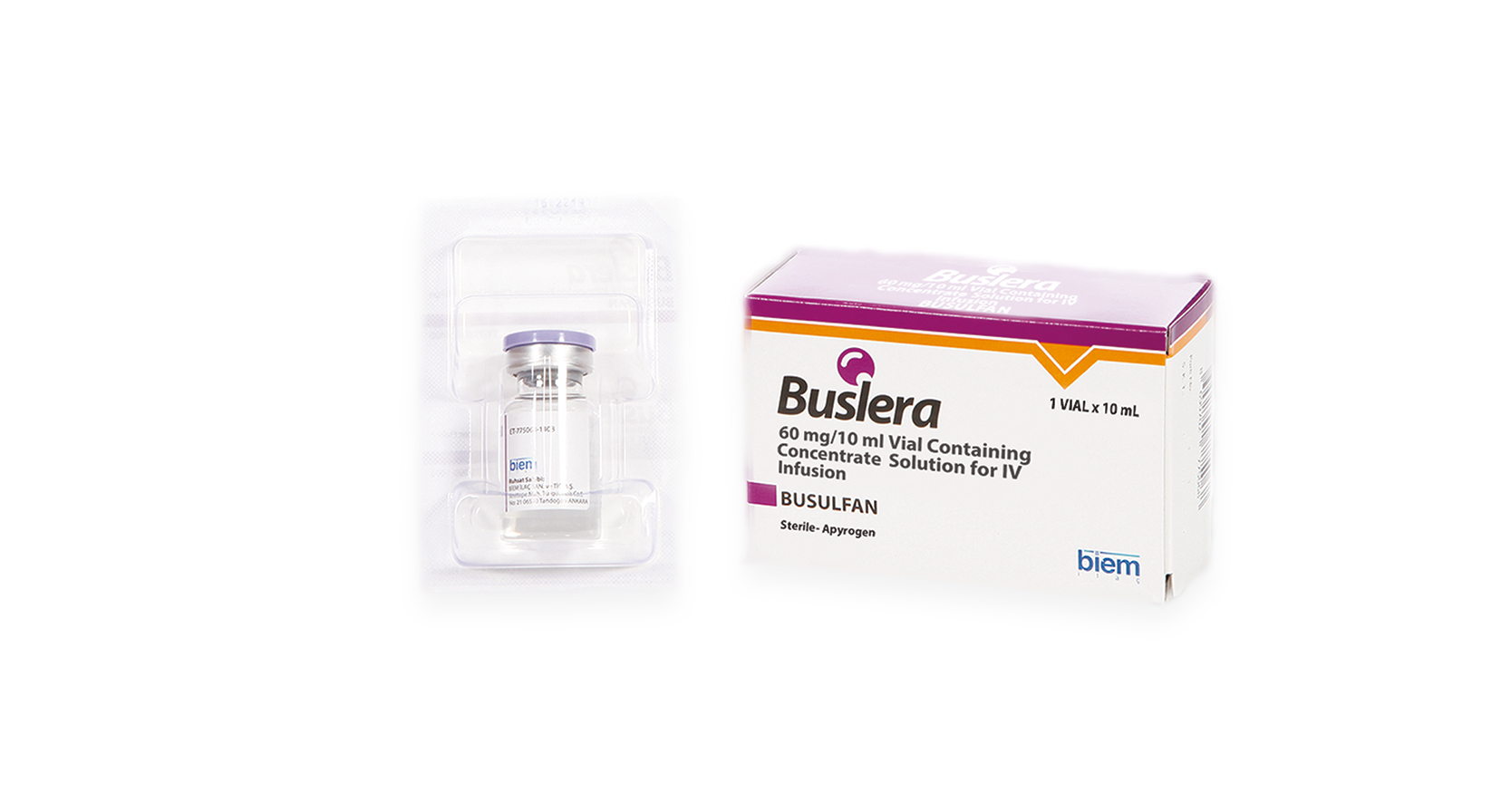















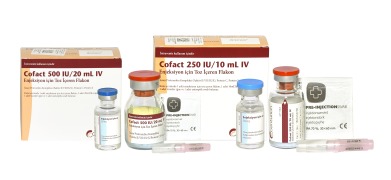












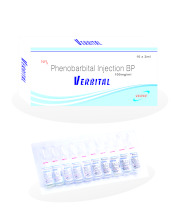
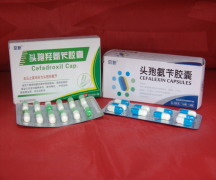









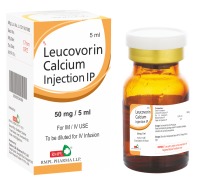















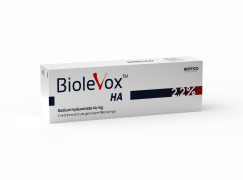



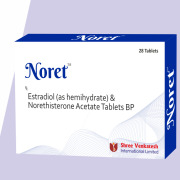

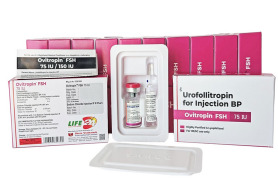

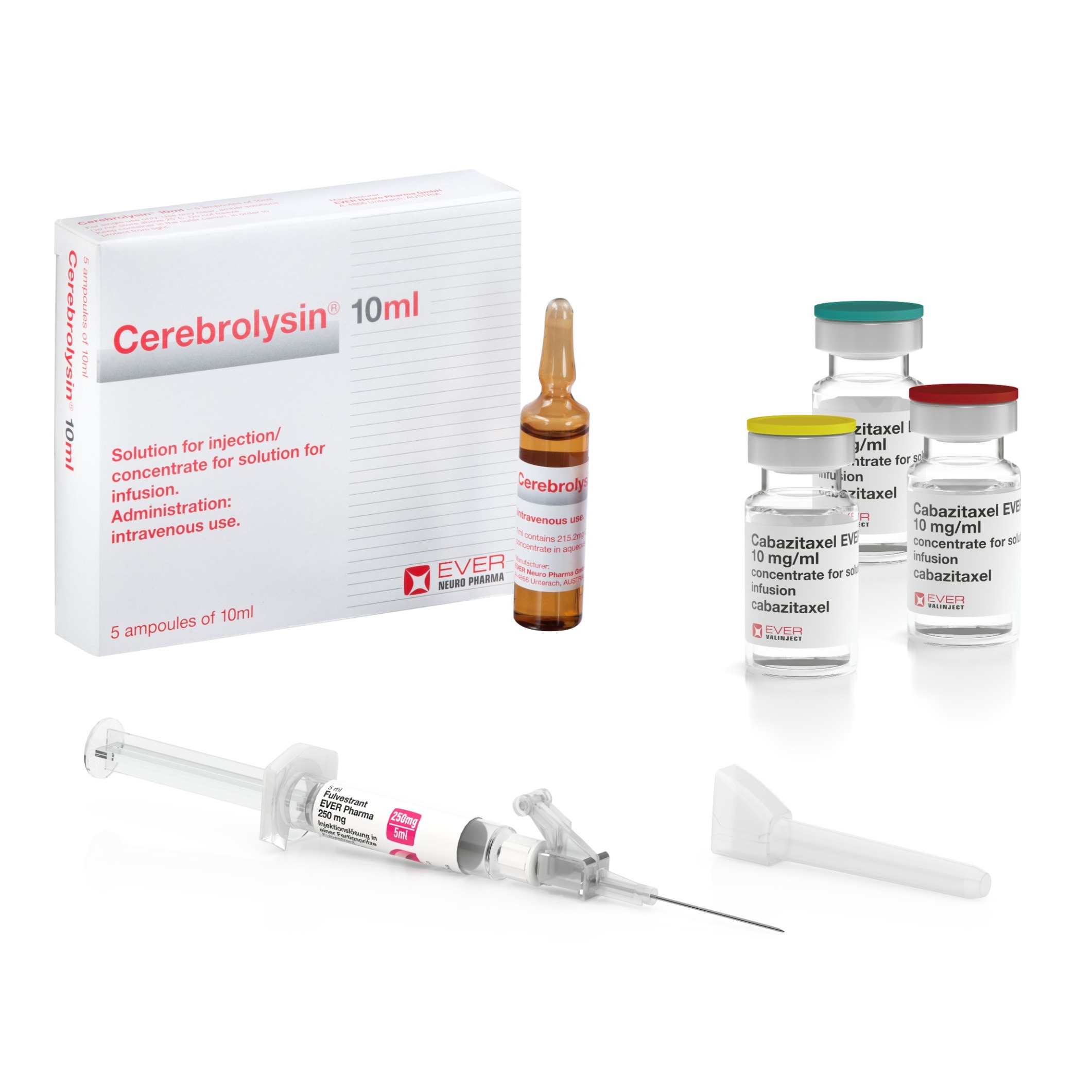


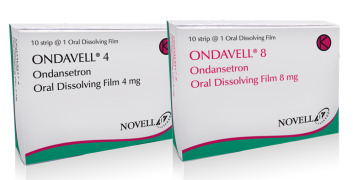
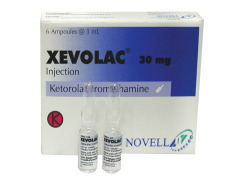















.png)







.png)

.png)



.jpg)


