Drug Product Fill-Finish Services
Product Description
INCOG BioPharma Services

-
US
-
2022On CPHI since
-
1Certificates
-
50 - 99Employees
Company types
Primary activities
Categories
INCOG BioPharma Services

-
US
-
2022On CPHI since
-
1Certificates
-
50 - 99Employees
Company types
Primary activities
More Products from INCOG BioPharma Services (1)
-
Product Analytical Services
Analytical Services: QC Chem/Microbio, stability, etc.
INCOG BioPharma Services resources (1)
-
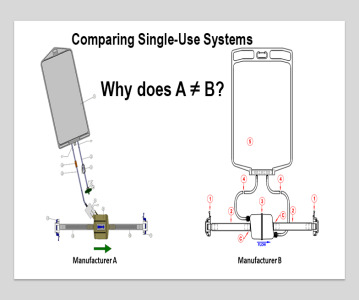
Sponsored Content Building resiliency into single-use systems
Jo Anne Jacobs, Director of Technical Services at INCOG BioPharma Services, discusses building a functional equivalence model for single-use manufacturing equipment and assemblies for use in drug product manufacturing.
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance