INCOG BioPharma Services
About INCOG BioPharma Services
Certifications
Categories

-
US
-
2022On CPHI since
-
1Certificates
-
50 - 99Employees
Company types
Primary activities
Meet us at
CPHI North America 2024
Pennsylvania Convention Center, Philadelphia
07 May 2024 - 09 May 2024
CPHI Milan 2024
Fiera Milano, Italy
08 Oct 2024 - 10 Oct 2024
Products from INCOG BioPharma Services (2)
-
Product Analytical Services
Analytical Services: QC Chem/Microbio, stability, etc.
-
Product Drug Product Fill-Finish Services
Fill-Finish Capabilities (product type) and Capacity:
Sterile liquid products
Biosafety level (BSL) 1, BSL 2 (OEL >= 10 µg/m³)
Vaccines (inactivated)
Current capacity: ~40M units/year
INCOG BioPharma Services Resources (1)
-
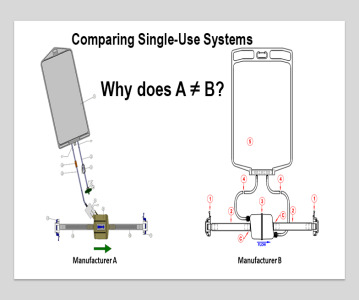
Sponsored Content Building resiliency into single-use systems
Jo Anne Jacobs, Director of Technical Services at INCOG BioPharma Services, discusses building a functional equivalence model for single-use manufacturing equipment and assemblies for use in drug product manufacturing.
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance