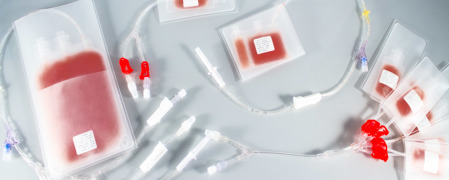
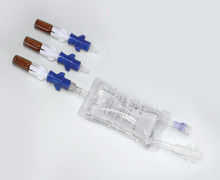
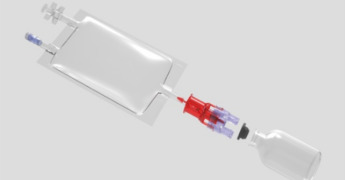
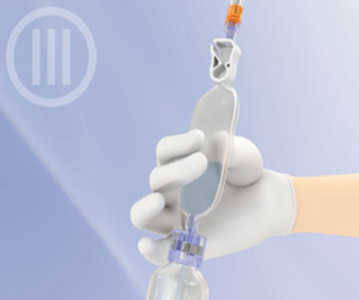
IV Bags

Product Description
Haemopharm Healthcare SRL
-
IT
-
2015On CPHI since
-
4Certificates
-
50 - 99Employees
Company types
Primary activities
Categories
Specifications
Haemopharm Healthcare SRL
-
IT
-
2015On CPHI since
-
4Certificates
-
50 - 99Employees
Company types
Primary activities
More Products from Haemopharm Healthcare SRL (15)
-
Product Washout: wound hydrotherapy
Washout is an ergonomic bag for the washing of critical wounds.
150ml, sterile, CE marked.
Main features:
- Injection point for adding active principles to the irrigation solution- Ergonomic shape of the bag which facilitates the controlled compression for the correct washing pressure on... -
Product Bags for cryopreservation of Stem cells and Tissues
SAFE2® is the brand line of the first double bag for cryopreservation up to -196°C, created to ensure the greatest safety for your bio samples, available in different volumes for both Stemcells (Stemchoice) and tissues (Tissuechoice) -
Product CSTD Ready-to-Transfer
Drug Reconstitution and intravenous infusion / intravesical instillation systems, intended for use in hospitals for drug preparation, designed to prevent the exposure of healthcare professionals to harmful effects of hazardous drugs. Allow easy dosing and eliminate the most pressing problems, such as needl... -
Product CSTD Ready-to-Use
CSTD (closed system drug transfer device) & CSSTD (closed system sterile drug transfer device).
Drug Reconstitution and intravenous infusion / intravesical instillation systems for various medicines, adhere to the principles of closed circuit, needle-free and contamination-free procedures, elimin... -
Product Freezing bags for Cryopreservation
SAFE® bags for storage of bone marrow, stem cells, umbilical cord blood in liquid nitrogen at a temperature of -196° C, made of special EVA material, with a wrapping bag and with customised label, can be flexibly customized to the needs of a biobank and transplant centres. Available 3D Bags technology. qq... -
Product Needle-free closures
Every day, healthcare professionals are exposed to deadly or toxic pathogens through contaminated syringe needles. Following the important FDA regulations and market trends, we provide needle-free systems that are safer for caregivers, preventing needle-stick accidents, and for patients, improving drug tra... -
Product Needle free connectors
Every day, healthcare professionals are exposed to deadly or toxic pathogens through contaminated syringe needles.
FDA regulations and market trends are moving towards needle-free systems that are safer for caregivers, preventing needle-stick accidents, and for patients, improving drug tr... -
Product NEW! Fillchoice® LUNG - CSTD
NEW! A filter-free Ready-to-Transfer CSTD with Smart Pressure Equalisation system for hazardous drug reconstitution and safe handling.
PATENT PENDING
The toxic effects of antineoplastic drugs used for cancer treatment, beyond the patient safety concerns, bring the occupational ris... -
Product NEW! Fillchoice® Refill KIT
A Ready-to-Transfer CSTD for drug reconstitution and administration to the patient from one or multiple drug vials.
• PATENDED In routine clinical practice of drug reconstitution, health care workers are at serious risk from the use of syringes with needles and exposure to the potential release o... -
Product Fillchoice® SIV
Ready-to-Use CSTD for a sterile connection between the administration bag and the vial.
• PATENDED If the sterility of a compounded drug preparation is compromised, a patient is at risk of infection, so operating conditions must prevent this.
Fillchoice® SIV is an innovative fin... -
Product Systems for Compounding Pharmacy
Haemopharm has developed a range of products for Pharmacy Compounding which consists of different systems for medication compounding, starting from bags for intravenous use in different sizes and configurations, sterile and ready for use. Compounding bags are available filled with sterile fluid or empty an... -
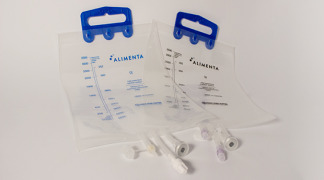
Product Alimenta TPN bags: new line made with a special material which improves the TPN bag features
Nutriline is the standard EVA TPN bags line, while Alimenta is the new line made with a special material which improves the TPN bag features:- Transparency: Alimenta is manufactured with a transparent film which allows an easy inspection of the solution contained in the bag. - PH stability: contrary to ...
Haemopharm Healthcare SRL resources (1)
-
News NEW! Fillchoice® LUNG - CSTD
NEW! A filter-free, ready-to-transfer CSTD with Smart Pressure Equalisation system for hazardous drug reconstitution and safe handling.
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance