End game strategies to reach patients globally and swiftly

Photo credit: Bristol Myers Squibb
The journey for new therapies is long; wondering if a proven therapy will be produced and distributed efficiently to reach the intended markets as soon as possible should not be another burden
Innovators around the world are investing time and resources in new therapies for unmet patient needs. The journey is long and arduous, wondering if their proven therapy will be produced and distributed efficiently to file NDAs and reach the intended markets as soon as possible should not be another burden.
The pandemic has heightened these concerns as reliable supply across the pharmaceutical and biotech industries have come under scrutiny. New drug sponsors are looking for partners with the right capability, capacity, and quality track record to launch their new drugs in major markets.
In response, WuXi STA has taken steps to assure our partners that their therapies will not be delayed by preventable bottlenecks. Our goal is to constantly provide our customers with better flexibility and greater confidence through increased capability and capacity with an enhanced global network and by enabling fast reliable commercial launch programs. This year we made advances on two fronts, first by expanding our global network so we have a more robust supply and second we developed a streamlined service package to ensure all necessary considerations are addressed and optimized when preparing for global NDA filing.
Capability and capacity with global reach
Our integrated CMC platform currently supports more than 1,500 new drug molecules including 47 phase III and 37 commercial projects. Globally we have supported more than 30 new drugs approved in recent years and our products have been launched in more than 105 countries. To stay ahead of our customer needs, we are always in capacity expansion mode by investing in both current and new facilities. In 2021, we announced major capacity expansions for both API and drug product. In 2 years, we will enhance our API capacity by about 3 times from 15 plants with 1400 m3 total reactor volume to 30 plants with 3900 m3 total reactor volume. From the drug product side, we acquired a commercial drug product manufacturing site in Couvet, Switzerland and announced the land purchase in Delaware, USA to build an integrated API and drug product manufacturing facility. The new acquisition brings our network of sites to 8 across Asia, North America and Europe.
The Couvet, Switzerland site, acquired from BMS, is a commercial drug product manufacturing site, constructed in 2016-2018 with state of art technologies and highest sustainability standards. In addition, the site’s EHS quality systems demonstrated a proven quality track record passing inspections from SwissMedic, USFDA, and Japan PDMA. Bringing this facility into our network allows greater confidence for our partners planning to reach European markets and beyond swiftly.
The new site we are building in Delaware, USA will be our largest campus on 190-acres designed to support both API and drug product pharmaceutical clinical and commercial manufacturing and will be our first API and drug product integrated site for both oral and injectable drugs. The site is expected to be operational by 2024 to further support our network in the US and globally.
Featured service package to streamline NDA filing at multiple agencies
Based on years of experience, WuXi STA created a unique streamlined drug product service package to facilitate fast commercial launch in global markets including the US, Europe and China.
The package brings together our global filing expertise, top-notch quality system, experienced teams, and high capacity to speed commercial readiness hence we named the package “Fast for Commercial Launch” (F4CL).
Two examples of how our F4CL program can be customized to meet client goals:
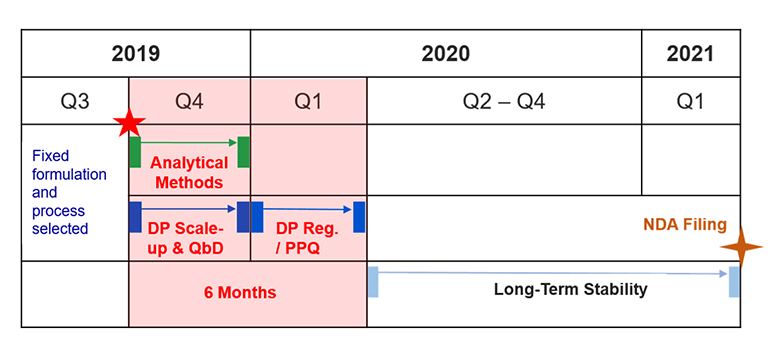
1. For China NDA filing: Registration batches and PPQ batches are combined to serve the purposes of collecting primary stability data and performing process performance qualification. The lead time from tech transfer to release of PPQ can be as short as 6 months. The example below enables 19 months for NDA filing with China NMPA.
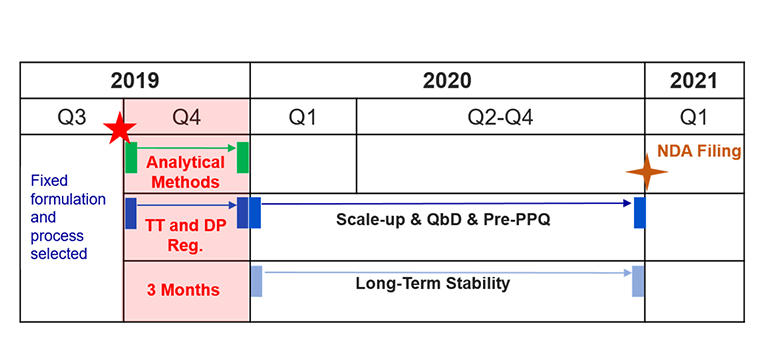
2. For US NDA filing: Registration batches are manufactured separately to kick off an early start of stability studies. Scale-up, QbD studies, and pre-PPQ batch(es) are performed while waiting for 12 months of stability results. This strategy allows more time for in-depth scale-up and QbD studies. The lead time from tech transfer to release of registration batches can be as short as 3 months.
In summary, WuXi STA has strengthened its integrated API and drug product CMC platform presenting our clients with greater options in Europe and global markets through an increased footprint, and expedited commercial launch through highly experienced teams providing the customized service package F4CL.

Related News
-
Sponsored Content Ashwagandha and Herbal Medicines: Pharma’s Next Big Opportunity
Herbal medicines and nutraceuticals have seen a surge in interest since the onset of the COVID-19 pandemic. Driven by patient interest in prioritising personalised and integrative medicines, the herbal ingredients industry is now faced with concerns pe... -
Sponsored Content Discover Our Organic Mineral Salts as APIs
Discover the range of organic mineral salts that serve as Active Pharmaceutical Ingredients available from Dr. Paul Lohmann®. -
Sponsored Content CPHI Podcast Series: Ursatec – celebrating 30 years of pioneering preservative free
In the latest episode of the CPHI Podcast Series, Digital Editor Lucy Chard spoke with Dominik Rocchi of Ursatec. -
Sponsored Content How healthcare trends inform dosage forms
Capsules encompass one of the most popular solid oral dosage forms for pharmaceutical products, with the global empty capsule market predicted to rise to USD $3.7 billion by 2026. The growth in the capsule market can be partly attributed to the many op... -
Sponsored Content 2023 Pharma Trend Outlook: Innovation, Resilience, and Pharma 4.0
Download our 2023 Pharma Trends Outlook report to discover the trends set to shape the pharmaceutical landscape in the new year, with expert opinions and insight from across the pharmaceutical value chain. -
Sponsored Content CPHI Podcast Series: Key Considerations in Selecting the Right CMO Partner
In this month's episode we hear from Jayna Blake, Senior Project Manager for Technical Programs at Baxter BioPharma Solutions, on key considerations for successful CMO selection. -
Sponsored Content Size doesn’t matter: How smaller deals are shaping healthcare M&A
Several mega-deals have made a splash in the pharma and life sciences industries over recent years – from AstraZeneca’s acquisition of Alexion for $39 billion to Gilead Sciences’ $21 billion purchase of Immunomedics. With am... -
Sponsored Content Rise in home-based healthcare shaping pharma packaging and drug delivery
The pharma packaging and drug delivery industry has long been synonymous with rapid innovation. As medicine advances and patient needs change, so too should drug packaging and devices. This has been particularly evident during the pandem...
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance

.png)