Laboratórios Basi: dedicated to high-quality development and manufacturing

Boasting a proud history of more than 50 years that has been built over a diversity of events, experiences and continuous learning, Laboratórios Basi is recognized as a key reference company in its sector.
Basi’s ambitious mission is to develop, manufacture, market and distribute medicines and therapeutic solutions globally, coined by the excellence of European quality, and supported by innovative technologies, in a competitive and flexible way.
Among its prime objectives are to be a global player in the pharmaceutical industry, to be a reference in fluid therapy, and to expand its presence in the Portuguese and Spanish markets.
The company has a vision of becoming the largest national medicinal products manufacturer and supports its activity via four key pillars: Flexibility, Innovation, Competitiveness and Efficiency.
Apart from an extended and diversified pharma portfolio of medicines, medical devices, food supplements, and dermocosmetics, Basi offers licensing and supply services for oral Liquids and semi-solid forms as well as contract manufacturing.
Pharma Portfolio
Medicines, Medical Devices, Food Supplements, and Dermocosmetics
Basi has more than 240 registered medicines in 17 different therapeutic areas and is present in more than 60 countries.
Licensing
- Oral Liquids and Semisolid forms
- Small Volume Parenterals
- A wide list of products with a complete document package (EU-CTD) available for licensing out including all regulatory support.
Contract Manufacturing
Basi has two manufacturing plants with a production capacity of over 300,000,000 units.
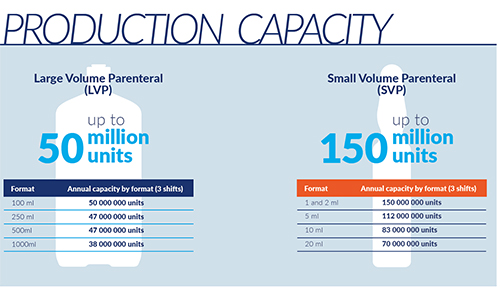
Smart Factory 4.0
- Liquid forms (syrups, oral solutions and suspensions, cutaneous solutions, etc.)
- Semisolid forms (creams, ointments, gels, enemas, etc)
- Small Volume Parenteral Solutions – glass ampoules
- Large Volume Parenteral Solutions – polypropylene bottles
Pharmaceutical Development
- Research, product development and innovative pharmaceutical forms in the health field.
- Formula and process development
- Tech transfer
Quality Control
- International reference laboratory, equipped with the latest analysis technologies.
- Physical and chemical tests
- Microbiological tests
- Stability studies
What are the key competencies that differentiate Basi from the competition?
Laboratórios Basi has recognized technical competence in the development of the most varied formulas, based on maximal use of the available technological means and an efficient installed capacity, making it an industrial player of reference with various international contracts and partnerships. Regarding operational capacity, BASI has an excellent fast response rate and believes in building serious long-term partnerships based on transparency.
What are the key areas that your research and development department are currently focused on?
Laboratórios Basi views Research and Development as a strategic area of the group, and is dedicated to a continued investment in research, product development and innovative pharmaceutical forms in the health field.
Research is supported by a research, development and innovation management system sustained by knowledge, supported by reference guidelines and standards, as well as by stimulating and motivating its employees to participate and collaborate in strategy and generation of new and promising ideas.
Research and development at Laboratórios Basi is committed to close collaboration with other R&D companies, as well as with internationally renowned R&D centers and Universities.
Currently, Laboratórios Basi has expansive collaborations with universities in several scientific areas.
What are the innovations that Basi has implemented to ensure its manufacturing is efficient, flexible and compliant with pharmaceutical quality requirements?
Basi has implemented Smart Factory 4.0 for Solutions for Injection at its facilities. This includes:
- a warehouse navigation system, HVAC real time monitoring, and WHI monitoring to enhance connectivity.
- robots to increase productivity
- connected objects for greater flexibility
- automated guided vehicles (AGVs) for increased efficiency

Can you share with us any exciting plans that the company has for the future?
Construction work on a branded new Center for the Research and Development Department will start by the end of 2022.

Related News
-
News On track at Pharmapack 2024 - The Track Sponsor interview: Gerresheimer
January 2024 brings both a new year and Europe’s leading packaging and drug delivery event. Gathering the world’s experts in pharmaceutical packaging together in Paris, France, Pharmapack 2024 offers exciting opportunities to learn and coll... -
News The Patient-Centric Synergy of Pharmaceutical CDMO and CRO Collaborations
Pharmaceutical collaborations are nothing new to the industry. Increasingly complex drug development programs, calls for supply chain resiliency, and the involvement of all key stakeholders throughout a drug’s development lifecycle are pushing co... -
Sponsored Content Discover Our Organic Mineral Salts as APIs
Discover the range of organic mineral salts that serve as Active Pharmaceutical Ingredients available from Dr. Paul Lohmann®. -
Sponsored Content CPHI Podcast Series: Ursatec – celebrating 30 years of pioneering preservative free
In the latest episode of the CPHI Podcast Series, Digital Editor Lucy Chard spoke with Dominik Rocchi of Ursatec. -
News Seqens Group Expands CDMO Offerings to Include CRDO
Meet with Seqens team members at CPHi, April 25-27, 2023 to learn how we have extended services to rapidly and sustainably advance drug development. VISIT CPHI Booth 441 -
News CDMO - Health is a Joint Project: Connect to Frankfurt on-demand
In this Connect to Frankfurt session, Emanuele Agnese, Business Development Manager and Pietro Allegrini, R&D Director at Indena, present the ways in which a reliable manufacturing partner in the pursuit of better health outcomes for patients and pharm... -
News Streamlined Development for Efficient Production of Bispecific Molecules: Connect to Frankfurt on-demand
In this Connect to Frankfurt session, Séverine Fagète, VP, Cell Line Development Services at Selexis (Geneva, Switzerland), and Brandon Brino (Durham, USA), Process Development Scientist and Group Leader at KBI Biopharma, present an overv... -
News MDR – How to collect post-market clinical follow-up data (PMCF): Connect to Frankfurt on-demand
In this Connect to Frankfurt session, Philipp Annecke, Junior Key Account Manager and Heinrich Martens, VP Regulatory Affairs of Fresenius Kabi (Bad Homburg, Germany) present guidances from the Medical Devices Regulation on clinical data evaluatio...
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance





.png)