Lyrus Life Sciences
About Lyrus Life Sciences
Certifications
Categories

-
IN
-
2019On CPHI since
-
4Certificates
-
250 - 499Employees
Company types
Primary activities
Meet us at
CPHI Frankfurt 2025
Messe, Frankfurt
28 Oct 2025 - 30 Oct 2025
Products from Lyrus Life Sciences (4)
-
Product Methenamine Hippurate Tabs 1000 mg
METHENAMINE is used to prevent urinary tract infections due to bacteria. It is not used to treat an active infection. It will not work for colds, flu, or other viral infections. -
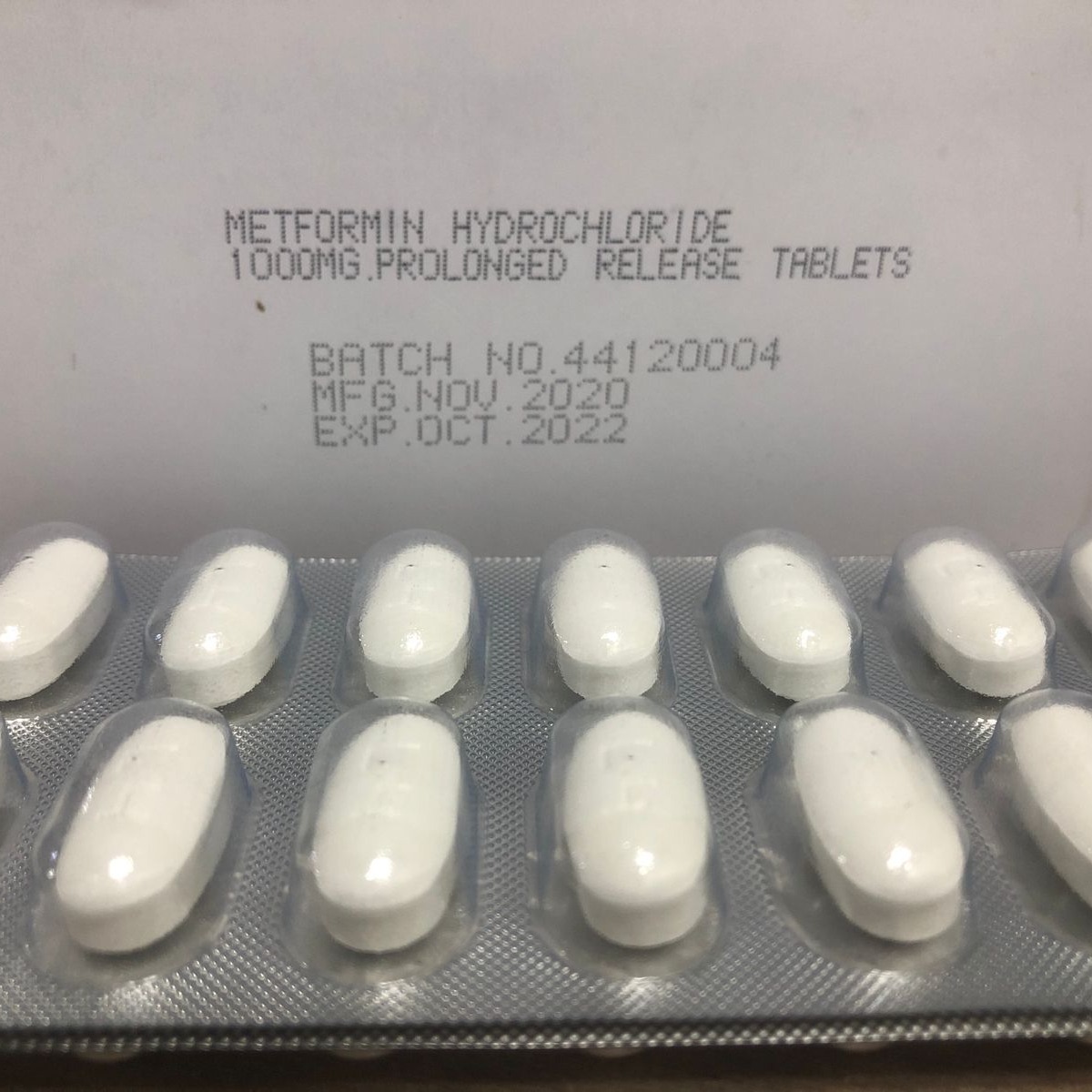
Product Metformin PR tablets
Metformin is used with a proper diet and exercise program and possibly with other medications to control high blood sugar. It is used in patients with type 2 diabetes. Controlling high blood sugar helps prevent kidney damage, blindness, nerve problems, loss of limbs, and sexual function problems. -
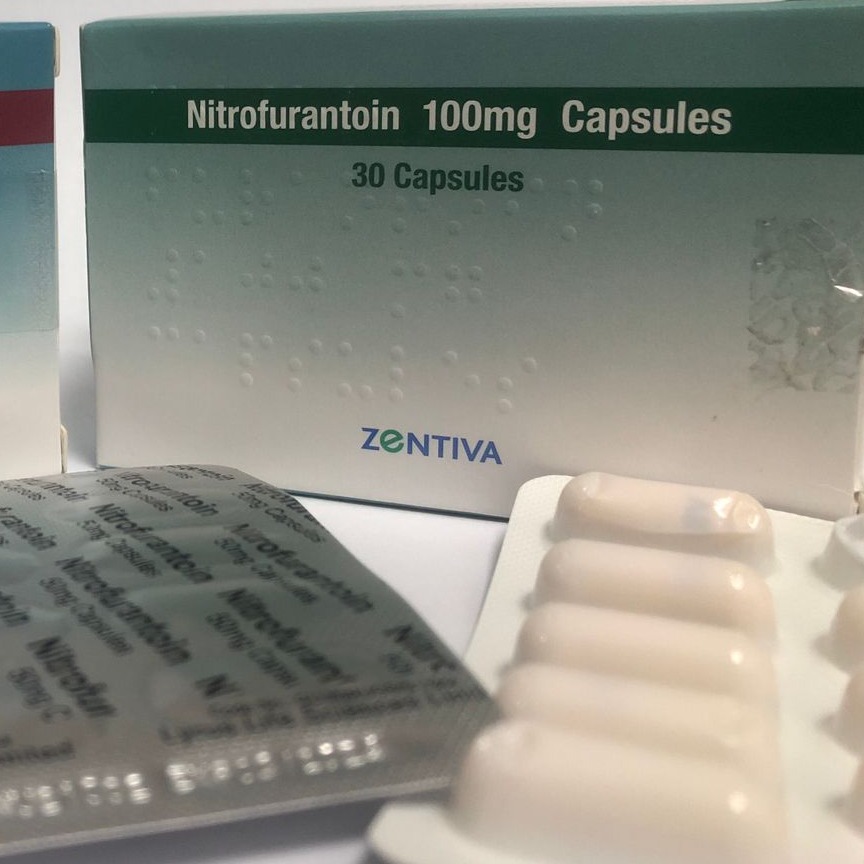
Product Nitrofurantoin Capsules
Nitrofurantoin is used to treat urinary tract infections. This medicine is an antibiotic. It works by killing bacteria or preventing their growth. -
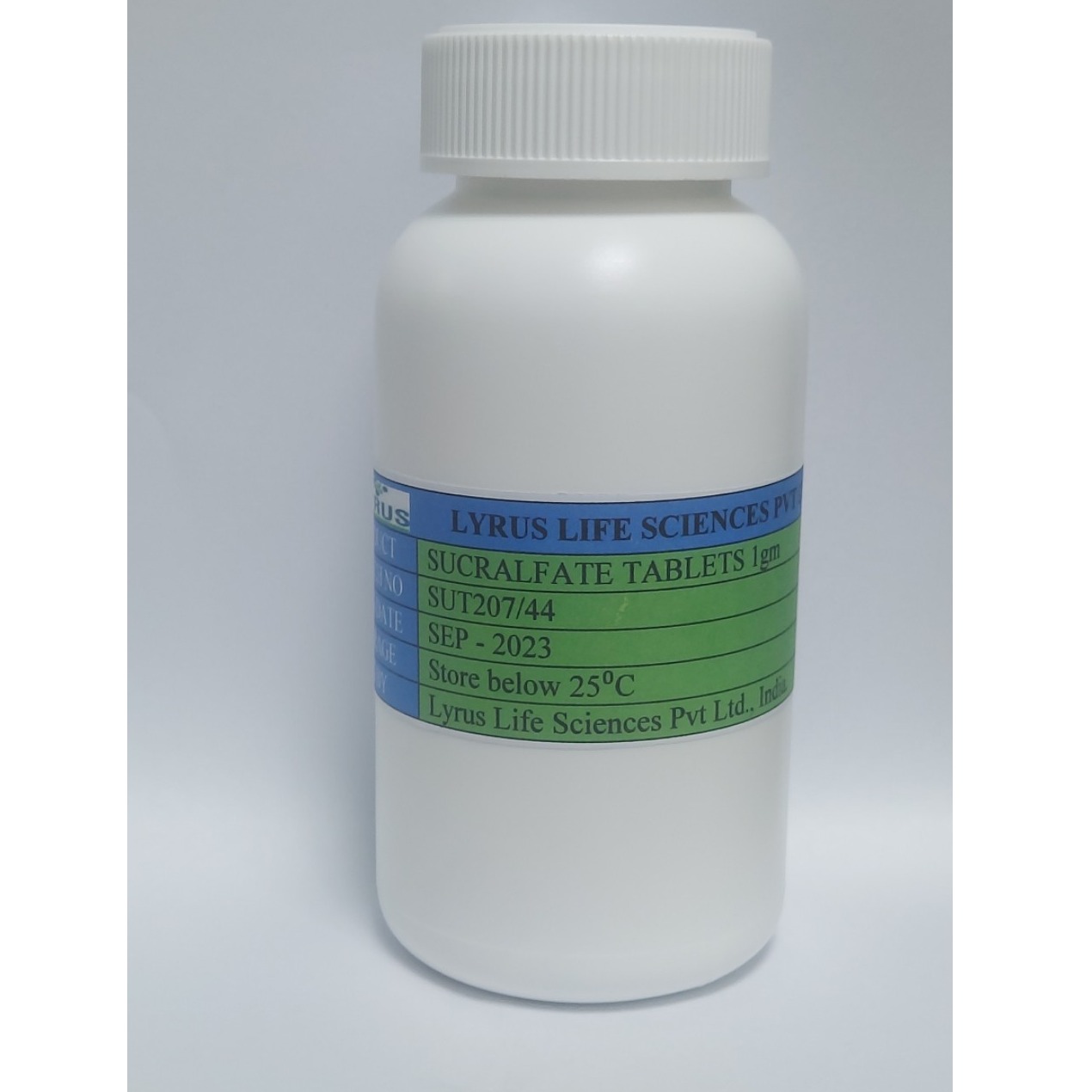
Product Sucralfate tablets
sucralfate, sold under various brand names, is a medication used to treat stomach ulcers, gastroesophageal reflux disease, radiation proctitis, and stomach inflammation and to prevent stress ulcers. Its usefulness in people infected by H. pylori is limited.
Lyrus Life Sciences Resources (2)
-
News Lyrus Receives US FDA approval for Critical UTI treatment in the US
Lyrus has received US FDA approval for our Abbreviated New Drug Application (ANDA) for 'Methenamine Hippurate Tablets USP, 1 gram', an AB-rated, substitutable generic version of Hiprex®.
-
Brochure Lyrus Life Science Brochure
Introduction:The Lyrus Life Science brochure provides a comprehensive overview of our company's capabilities, management profile, research and development strengths, and production facilities. It serves as a valuable resource to gain insights into our operations and the therapies we specialize in. At Lyrus Life Science, we are committed to developing innovative products using non-infringing technologies, revolutionizing the healthcare industry.
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance