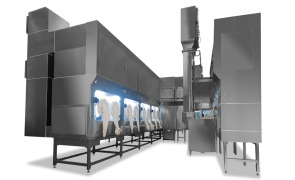
Sterilization Systems

Product Description
Telstar

-
ES
-
2016On CPHI since
-
1Certificates
-
500 - 999Employees
Company types
Primary activities
Categories
Specifications
Telstar

-
ES
-
2016On CPHI since
-
1Certificates
-
500 - 999Employees
Company types
Primary activities
More Products from Telstar (2)
-
Product GMP Production Freeze Dryers
Telstar has been designing and manufacturing freeze dryers for the pharmaceutical industry for over 50 years.
Improvements in the process of Freeze drying and of the equipment to carry it out have been a continuous challenge since Telstar’s very beginnings. The design of units for e... -
Product Containment & Aseptic Barrier Isolator Systems
n the global pharmaceutical market with the ever growing demands on materials, operator and environmental protection, consideration toward increasing standards of safety, sterility assurance and regulatory compliance has never been higher.
As applications expand into less traditional are...
Telstar resources (2)
-
News Telstar’s centre in the UK consolidates its growth by supplying integrated isolation tech solutions for aseptic and containment pharma production
Telstar’s centre in the UK consolidates its growth by supplying integrated isolation tech solutions for aseptic and containment pharma production -
Whitepaper Optimizing equipment performance by smart maintenance platform
Download the latest Whitepaper from Telstar on how acquiring equipment real-time data and defining new maintenance strategies reduce eventual breakdowns and prevent downtime of the final user assets.
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance