Study finds 88% of pharma companies base global launch planning on regulatory affairs guidance
Various tools help drug companies establish successful global launch plans.
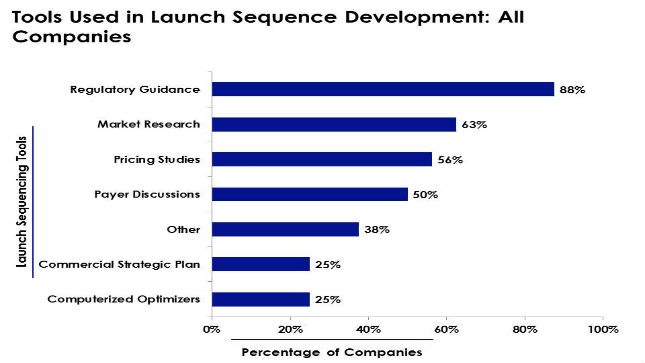
A new benchmarking study published by Cutting Edge Information has discovered that regulatory guidance is the most common tool that pharmaceutical companies use when developing their global launch strategies.
In fact, the majority, 88% of surveyed firms, look to regulatory guidance to inform global launch planning. Furthermore, 83% of large pharmaceutical companies and 80% of small pharmaceutical companies that participated in the study rely on regulatory guidance to establish a launch sequence. Regulatory guidance educates market access teams regarding potential requirements that companies may face when entering specific markets.
The study, Pharmaceutical Launch Sequencing: Establishing Patient Access and Understanding Global Pricing, researched the impact of various functional departments on launch sequence planning. For example, the study found that 63% of surveyed companies use market research to help their launch sequences. Surveyed drug companies used both in-house and outsourced market research to make decisions. Companies used other when developing launch sequences, such as pricing studies, payer discussions, commercial strategic plans, and computerized optimizers.
"Preparing a successful global launch is a challenge for any multinational drug company," said Adam Bianchi, senior director of research at Cutting Edge Information. "That's why it is important to use regulatory guidance and market research throughout the process."
Adequately staffed cross-functional boards are able to compile key commercial, regulatory and market access insights which influence these plans. Companies must also be able to resource these teams with adequate launch sequencing budgets and the tools necessary to make informed decisions.
Pharmaceutical Product Launch Sequencing: Maximizing Revenue and Achieving Global Market Access helps companies to maximize their margins when launching products around the world. The report identifies key trends in how companies approach launch sequences and what drives decision making among internal and external stakeholders. The report's key findings include
Related News
-
News Patients vs Pharma – who will the Inflation Reduction Act affect the most?
The Inflation Reduction Act brought in by the Biden administration in 2022 aims to give better and more equitable access to healthcare in the USA. However, pharma companies are now concerned about the other potential costs of such legislation. -
News CPHI Podcast Series: What does the changing US Pharma market mean for industry and patients alike?
In this week's episode of the CPHI Podcast Series Lucy Chard, Digital Editor for CPHI Online is joined by James Manser to discuss the political and market changes in the US pharma field. -
News CPHI Barcelona Annual Report illuminates industry trends for 2024
The CPHI Annual Survey comes into it’s 7th year to report on the predicted trends for 2024. Over 250 pharma executives were asked 35 questions, with their answers informing the industry landscape for the next year, spanning all major pharma marke... -
News Which 10 drugs are open to price negotiation with Medicare in the USA?
The Centres for Medicare & Medicaid Services, under the Biden administration in the USA, has released a list of the 10 drugs that will be open to price negotiations as part of the new legislation under the Inflation Reduction Act (IRA). -
News EU Medical Devices Regulation causes unintended disappearances of medical devices for children, doctors state
Doctor groups and associations have appealed to the EU to correct the EU Medical Devices Regulation law that may cause unintended shortages of essential drug and medical devices for children and rare disease patients. -
News 10 Major Drug Approvals So Far in 2023
Last year, 37 novel drugs were approved by the FDA, this was a high number for such a category, and covered many fields including oncology, demonstrating how promising further research is, and how it is only continuing to build. To date, there are alre... -
News Detecting Alzheimer's disease with a simple lateral flow test
A novel rapid diagnostic test for early-stage Alzheimer's disease has been developed using a biomarker binder from Aptamer Group along with technology from Neuro-Bio, the neurodegenerative disease experts. -
News CPHI Podcast Series: outsourcing and manufacturing trends
Listen to the CPHI Podcast Series this June to hear Gil Roth of the PBOA speak with Digital Editor Lucy Chard about the biggest trends and topics to watch in pharma outsourcing and manufacturing at the minute.
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance







