Technical Barrier of Sodium Hyaluronate Pharma Grade: "Bacterial Endotoxins "
The bacterial endotoxins level of Topscience is around 0.001 IU/mg, which was 50 times lower than that specified in Ph. Eur. For bacterial endotoxins control of Sodium Hyaluronate, Topscience is in the leading position in the industry.
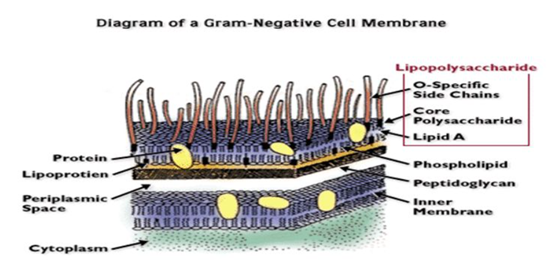
Bacterial endotoxins, also known as
lipopolysaccharide, is the main structure of the outer membrane of
Gram-negative bacteria. It will fall off from the outer membrane of bacteria
and be released into the surrounding media during the various stages of
bacterial growth and reproduction as well as when the bacteria are autolyzed or
lysed.
Topscience has been working on lower levels of bacterial endotoxins. According to the bacterial endotoxins test results of Sodium Hyaluronate in successive batches, the bacterial endotoxins level of Topscience is around 0.001 IU/mg
Topscience has now upgraded the in-house quality standard of Sodium Hyaluronate injection grade, and the upper limit of bacterial endotoxins has been updated to 0.005 IU/mg, which confirms the high-quality assurance of Topscience.
The level of bacterial endotoxin of Topscience is 50 times lower than the standard that specified in Ph. Eur. which is due to the strict and efficient control of the product quality from the collection of raw materials to products release. Topscience' Sodium Hyaluronate is produced by microbial fermentation without any raw material from animal, and exellent impurity control including bacterial endotoxin, heavy metal, elemental impurity, microbial contamination, etc.,


Related News
-
Sponsored Content Ashwagandha and Herbal Medicines: Pharma’s Next Big Opportunity
Herbal medicines and nutraceuticals have seen a surge in interest since the onset of the COVID-19 pandemic. Driven by patient interest in prioritising personalised and integrative medicines, the herbal ingredients industry is now faced with concerns pe... -
Sponsored Content Discover Our Organic Mineral Salts as APIs
Discover the range of organic mineral salts that serve as Active Pharmaceutical Ingredients available from Dr. Paul Lohmann®. -
Sponsored Content CPHI Podcast Series: Ursatec – celebrating 30 years of pioneering preservative free
In the latest episode of the CPHI Podcast Series, Digital Editor Lucy Chard spoke with Dominik Rocchi of Ursatec. -
Sponsored Content How healthcare trends inform dosage forms
Capsules encompass one of the most popular solid oral dosage forms for pharmaceutical products, with the global empty capsule market predicted to rise to USD $3.7 billion by 2026. The growth in the capsule market can be partly attributed to the many op... -
Sponsored Content 2023 Pharma Trend Outlook: Innovation, Resilience, and Pharma 4.0
Download our 2023 Pharma Trends Outlook report to discover the trends set to shape the pharmaceutical landscape in the new year, with expert opinions and insight from across the pharmaceutical value chain. -
Sponsored Content CPHI Podcast Series: Key Considerations in Selecting the Right CMO Partner
In this month's episode we hear from Jayna Blake, Senior Project Manager for Technical Programs at Baxter BioPharma Solutions, on key considerations for successful CMO selection. -
Sponsored Content Size doesn’t matter: How smaller deals are shaping healthcare M&A
Several mega-deals have made a splash in the pharma and life sciences industries over recent years – from AstraZeneca’s acquisition of Alexion for $39 billion to Gilead Sciences’ $21 billion purchase of Immunomedics. With am... -
Sponsored Content Rise in home-based healthcare shaping pharma packaging and drug delivery
The pharma packaging and drug delivery industry has long been synonymous with rapid innovation. As medicine advances and patient needs change, so too should drug packaging and devices. This has been particularly evident during the pandem...
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance
.png)