Venus Pharma GmbH
About Venus Pharma GmbH
Certifications
Categories

-
DE
-
2019On CPHI since
-
5Certificates
-
1000 - 4999Employees
Company types
Primary activities
Meet us at
CPHI Frankfurt 2025
Messe, Frankfurt
28 Oct 2025 - 30 Oct 2025
Products from Venus Pharma GmbH (5)
-
Product ANTIBIOTIC INJECTABLES Meropenem(FOR CRITICAL CARE)
CEFEPIME FOR INJECTION | CEFTRIAXONE | CEFTAZIDIME FOR INJECTION | CEFOPERAZONE + SULBACTAM FOR INJECTION | CEFUROXIME FOR INJECTION | CEFOTAXIME FOR INJECTION | CEFTAZIDIME AVIBACTAM FOR INJECTION | CEFTRIAXONE SULBACTAM FOR INJECTION | ELORES: CEFTRIAXONE, SULBACTAM & EDTA FOR INJECTION | AZTREONAM F... -
Product ONCOLOGY LIQUID INJECTABLES
CARBOPLATIN | CISPLATIN | CYTARABINE LIQUID | DOCETAXEL WITH SOLVENT | DOCETAXEL (CTD) SINGLE VIAL | DOXORUBICIN | EPIRUBICINE | ETOPOSIDE | IRINOTECAN | LEUCOVORIN CALCIUM | METHOTREXATE | OXALIPLATIN | PLERIXAFOR | PACLITAXEL | VINCRISTINE | 5-FLOUROURACIL | FULVESTRANT
-
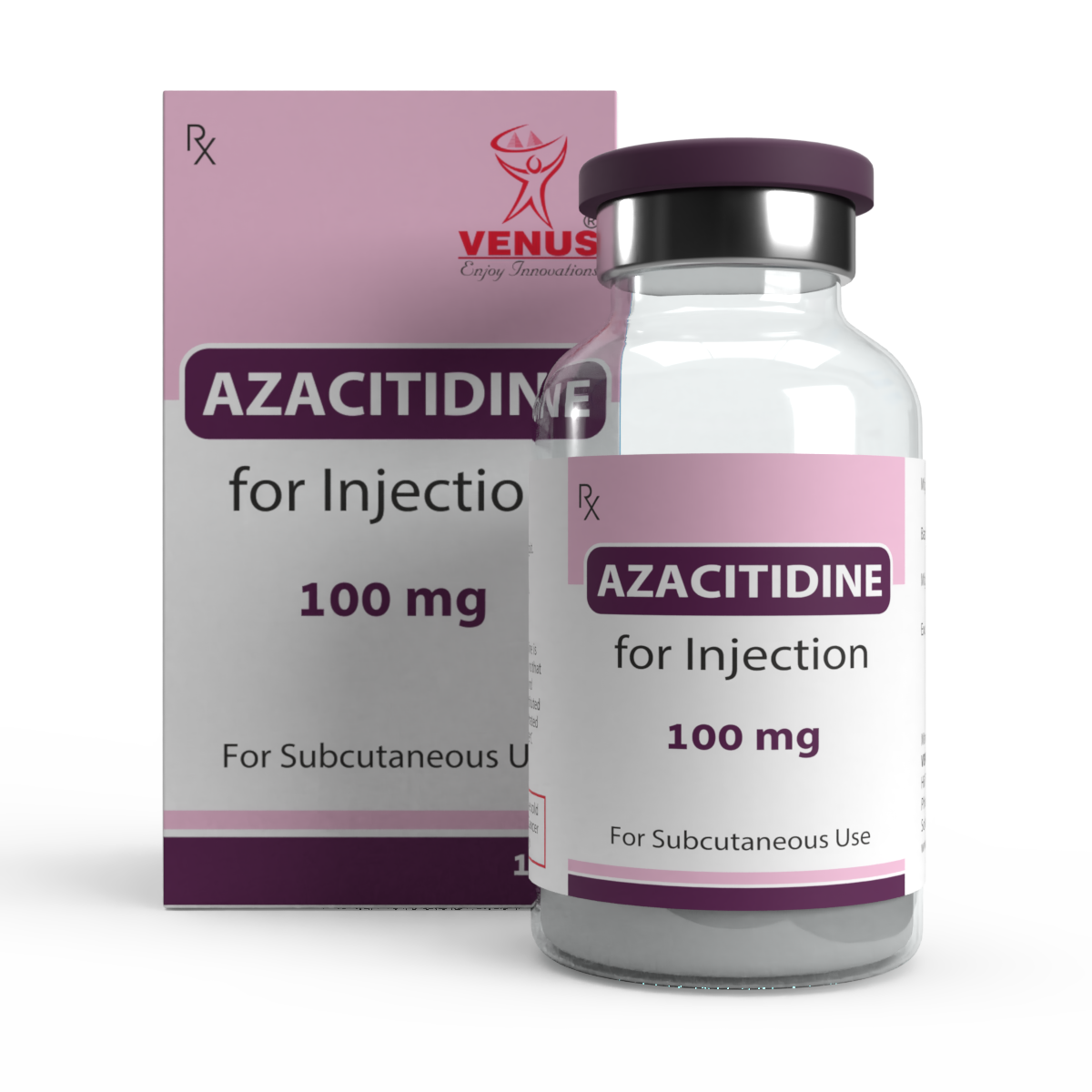
Product ONCOLOGY LYOPHILIZED INJECTABLES
AZACITIDINE | BENDAMUSTINE | BLEOMYCIN | BORTEZOMIB | CYTARABINE | DACARBAZINE | EPIRUBICIN | GEMCITABINE | PEMETREXED | TOPOTECAN | ZOLENDRONIC ACID
-
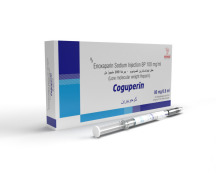
Product PREFILLED SYRINGE (ENOXOPARIN) BIOSIMILAR
ENOXAPARIN PFS 100MG/ML
-
Product Product List for Venus Remedies Limied-CPHI 2024
At VENUS REMEDIES LIMITED, we approach business partnerships with a strategic mindset, fostering collaboration that is not only successful in the short term but also sustainable and mutually beneficial in the long run.
Venus Pharma GmbH Resources (2)
-
News Venus Pharma Product list- CPHI Milan
Venus Pharma Product list- CPHI Milan
-
Brochure Stay tuned for more exciting updates!
Venus Remedies is making waves in the healthcare industry once again. We're bringing our latest groundbreaking innovations and global collaborations to the hear
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance