Custom Manufacturing of Dosage Form Drugs
Custom Manufacturing of Dosage Form Drugs Companies (46)
Custom Manufacturing of Dosage Form Drugs News
-
News CPHI Frankfurt 2022: Innovator Interview - Lonza Small Molecules
In this series of interviews, we speak to companies on the CPHI Frankfurt show floor who are driving innovation in pharma for a better healthcare future. Here, we spoke to Henny Zijlstra, Senior Director, Commercial Development at Lonza Small Molecules... -
News Opioid antagonist naloxone considered safe for over-the-counter use
The US FDA have approved the distribution of naloxone, a drug that reverses opioid overdose, in an over-the-counter setting, in some dosage forms. -
News Partnership between Bora Pharmaceuticals and TaiRx, Inc. for anticancer drug manufacturing
A partnership between Bora Pharmaceuticals and TaiRx, Inc. is expected to support the manufacturing of breakthrough anticancer drug CVM-1118, with two phase II clinical trials to commence. -
News Catalent fills gap in the market with new development offering, Xpress Pharmaceutics
Xpress Pharmaceutics will facilitate adaptive clinical trials and reduce the time taken to complete FIH studies
Custom Manufacturing of Dosage Form Drugs Products (106)
-
Product Highly Potent API Handling
HPAPI product handling from concept to commercial-scale manufacturing
Utilizing a combination of technology, infrastructure and expertise, we provide you with seamless, flexible HPAPI development and manufacturing tailored to your specific needs and resulting in reduced program timelin...
-
Product CordenPharma Highly Potent & Oncology Platform
One Source for Highly Potent & Oncological Products
• Integrated Supply: APIs & Oral / Sterile Drug Products • State-of-the-art facilities able to handle API and Drug Product of the highest potency • Development, clinical trial and commercial manufacturing • Full-service offering includi...
-
Product Formulation Development including Inhaled Products
Inhaled products and formulation development: The development of drugs using experimental design approaches with integrated stability testing and storage is available from us. Our formulation specialists can provide a wide range of formulations in a timely and cost-effective manner in order to identify t...
-
Product Cannabidiol (CBD) Softgels
We offer fully assistance for development, formula optimization, and encapsulation of CBD-THC products. Soft gelatin capsules are the ideal dosage form considering both bioavailability and stability of Cannabis medicinal products. Additionally, our unique and patenteted Unigel technology offers a unique pl...
-
Product Betahistine 12.5 mg/ml Oral Drops
Glass bottle containing 100 ml oral solution with disposal pump.
-
Product Specialty Commercial Manufacturing Solutions
Experic provides a variety of services to support Phase 3 to commercial-scale process transitions to create a customized solution for your specialty, niche, orphan, or combination product, or support novel pharmaceutical particle engineering technologies. Build-to-suit dedicated suites, a flexible...
-
Product Dossier - LyoDis (ODT ) Freeze Drying tablets
LyoDis -ODT - Lyophlized Freeze drying tablets - various development dossier available for the scale-up for EU and other global markets

-
Product Device CDMO
Sanner Group offers a vaste portfolio of capabilities for design, development, and manufacturing of medical devices for regulated markets. Being recognized as a smart, agile and reliable contract development and manufacturing organization (CDMO), we deliver highest quality and customers trust in our long-t...
-
Product Isotretinoin soft caps
GAP is offering for out licence ISOTRETINOIN soft caps 5-10-20-40mg with the most updated BE study (2018)
GAP manufacturers per year over 250 milion caps of Isotretinoin Product is offered for licensing or contract manufacture agreement
-
Product Clinical Manufacturing
.Integrated drug development service (CRO/CDMO) including clinical manufacturing and management.
The range of activities offered by Bluepharma includes:
- Clinical material cGMP manufacturing
- Clinical supply management
- clinical material
- placebo
- ...
-
Product Sterile Injectables Contract Manufacturing
Pfizer CentreOne is a global leader in sterile injectables fill-finish.Count on us to carefully guide your compound from development through launch. We’re known for our expertise in: • Complex biologics • Controlled substances • Sterile suspensions • Lyophilization
You’ll be supported not on...
-
Product Hot Melt Extrusion
To successfully develop and manufacture your polymer-based delivery systems, like implants and intra-vaginal rings, we have advanced extrusion capabilities in Malmö (Sweden) and Putnam (US Connecticut). The US site is also a center of expertise for Hot Melt Extrusion (HME).
HME improves the bioa...
-
Product OTF - Oral Thin Films
Oral thin films are loaded with active substances. The Thin films are taken orally and dissolve immediately in the mouth or are applied to the mucosa. For transmucosal films, the active substance enters the bloodstream directly via the oral mucosa, without having to first pass through the gastrointestinal ...
-
Product Pre-mixed IV solutions: bags
Premixed solutions in customized IV bags, sizes: 50, 100, 250, 500 and 1000 ml (one port, two ports, twist-off, luer valve)
Grifols Partnership is specialized in developing and manufacturing high quality sterile solutions in polypropylene flexible bags in different sizes to suit every need in both ...
-
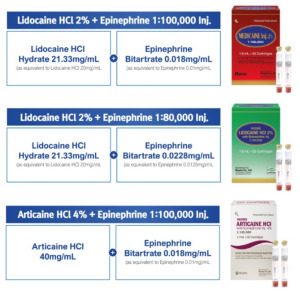
Product Huons Dental Cartridges
Lidocaine HCl 2% + Epinephrine 1:100,000 Inj. Lidocaine HCl 2% + Epinephrine 1:80,000 Inj. Articaine HCl 4% + Epinephrine 1:100,000 Inj.
Cartridge Ampoule packed Dental Anesthetics
Currently exporting to more than 30 countries in the world.
Looking for a capable importer/dist...
-
Product Solid & Liquid Dose Drug Manufacturing & Development
From OTC and Rx to diagnostics and dietary supplements, Avéma manufactures a full range of solid and liquid dose products, all manufactured under strict FDA guidelines and cGMP compliance. With an ever-growing portfolio of innovative formulas and a diverse mix of state-of-the-art equipment, our offerings a...
Upcoming Events
-
CPHI North America 2024
Pennsylvania Convention Center, Philadelphia
07 May 2024 - 09 May 2024 -
CPHI & PMEC China 2024
Shanghai New International Expo Center
19 - 21 June 2024 -
CPHI South East Asia 2024
Queen Sirikit National Convention Center, Bangkok, Thailand
10 Jul 2024 - 12 Jul 2024
Pharmaceutical Industry Webinars
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance













































.png)
.png)







.jpg)
.jpg)






















































































































.png)


.png)



.jpg)