Flamma S.p.a.
About Flamma S.p.a.
Certifications
Categories

-
IT
-
2015On CPHI since
-
3Certificates
-
500 - 999Employees
Company types
Meet us at
CPHI Milan 2024
Fiera Milano, Italy
08 Oct 2024 - 10 Oct 2024
Products from Flamma S.p.a. (5)
-
Product API manufacturing (pre-clinical + early phase)
Flamma USA is located in Malvern, PA and can help with early-stage pre-clinical supply of small molecule APIs -
Product Fmoc-Amino acids
Flamma is a premier supplier of custom made specialty Fmoc- amino acids. Please stop by to learn more -
Product Small peptides
Flamma can make small peptide fragments via liquid phase synthesis. Stop by to learn more. -
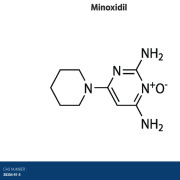
Product Minoxidil
Flamma Spa offers minoxidil so please reach out to learn more directly via FlammaGroup.com -
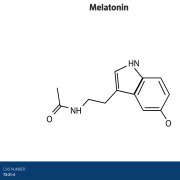
Product Melatonin
Flamma Spa the highest quality melatonin on the market so please reach out to learn more directly via FlammaGroup.com
Flamma S.p.a. Resources (2)
-
Sponsored Content Flamma Group acquires additional API site in Italy
The company wil acquire a third API manufacturing site in Lecco, Italy as of April this year -
Brochure FLAMMA Overview
FLAMMA is a CDMO with Headquarters near Milan, Italy. As a fully integrated CDMO, we can leverage our 100% self-owned, self-managed facilities, in Italy and China to deliver a stable, reliable supply chain. We specialize in the cGMP manufacturing of APIs, NCEs, RSMs, &Advanced intermediates (NCEs and generics). We use our expertise in high value chiral materials (specifically amino acid related materials) to provide solutions to customers who not only have need for chiral materials but also clients who have other requirements with a various type of chemistries. In addition, we also manufacture specialty compounds and advanced intermediates for the nutraceutical and cosmetic industries, and others.
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance