Method Development
Method Development Companies (31)
Method Development News
-
News Regis completes expansion of US-based API development facility
The CDMO doubles its capacity to take on new development projects -
News Quality laboratory data with PPD Laboratories
Quality laboratory data is vital to your pharmaceutical development program. -
News Recipharm launches new service to support companies with QC and analytical requirements
Services including method development, method validation, and stability program design and implementation. -
News High demand encourages Sterling Pharma's phased expansion of its US facility
The investment will allow the CDMO to take on new projects, as well as bolster the company's chemistry development capabilities.
Method Development Products (59)
-
Product Development and Validation of Analytical Methods
Analytical methods development and validation: Our method development scientists work with a broad range of products, methods, and analytical technologies (chromatography, mass spectrometry, spectroscopy, biophysical analysis, bioanalytical techniques, etc.) to ensure that the method will meet its int...
-
Product Analytical services - OEL: 50 ng/m3
• Control of pharma samples solid state : Identification and quantification of solid state, method development and validation, routine QC. • Analytical techniques available : XRPD, DSC, TGA, DVS, Laser PSD, FTIR, IDR. • All analyses are applicable to toxic samples down to : 50 n...
-
Product Scale-Up and Transfer of lyophilization processes
From the laboratory to the industry.
The key to a correct Scale-Up and Transfer of lyophilization processes is the knowledge of the developed product, as well as the control of the process and the capabilities of the final equipment.
-
Product Analytical package
XEDEV provides a broad range of analytical tools including : • Solid state analysis: mDSC, XRPD, PLM, TGA, FTIR, RAMAN • Powder characterisation : SEM, PSD (both wet and dry), flowability • Tablet characterisation : friability, disintegration, hardness, dissolution • Residual solvent determination :...
-
Product Biocompatibility & Toxicology Testing
Nelson Labs offers a full trifecta of services to meet the requirements of ISO 10993 for Extractables & Leachables, Biocompatibility and Toxicological Assessments of medical devices. Biocompatibility testing is very common in the medical device industry. However, with 24 possible categories, each with...
-
Product Development
Synerlab provides wide range of services which includes development. Contact us for more information.
-
Product QACS Pharmaceutical services
QACS Lab is GMP/GLP certified and provides contract laboratory testing for Pharmaceuticals. Methodologies stay in accordance with EMA & FDA. QACS is equipped with Microbiological - Chemical - Molecular - Packaging laboratory premises to provide variety of Pharmaceutical testing solutions &...
-
Product Innovation & Formulation
Innovation & Formulation: We offer pharmaceutical R&D, feasibility studies, proof-of-concept, formulation development and analytical method development for multiple dosage forms such as solids, semi-solids, liquids, oral films, and transdermal patches. We can work with over 30 pharma technologies f...
-
Product Complex drug formulation
We like to get involved right from the start of a development project and work closely together with our clients and partners on transforming the idea into a product with a solid and commercially viable target product profile (TPP). We set the bar high but always work step by step with a clear focus on...
-
Product Analytical Services
Pharmaffiliates is providing all the Analytical Services (i.e. Method Development, Method Validation & Transfer, Stability Studies, Contract Analysis, etc)
-
Product Development Service
Lomapharm GmbH provides wide range of services which includes development service. It provides the whole range of control services - from raw material testing to stability testing according to GMP and ICH regulations. One of the main areas of competence and experience is also available for galenic dev...
-
Product Drug Development Services
Aurigene Pharmaceutical Services offers a wide range of chemical development services for pharmaceutical, agrochemicals, fine chemicals and animal health industry. We support the development of Key Starting Materials (KSM) / Registered Starting Materials (RSM), advance intermediates and APIs from the ...
-
Product Medical Devices for various medical procedure (HA injection syringes accessories, Blood analysis, surgical tool, orthopedics...)
De l'idée à la conception, l'industrialisation jusqu'à la qualification et la production.
From the idea trough the design, industrialization until qualification and production.
-
Product Automated Leak Testing for Sterile Pharmaceuticals
Our automated CCI testers for vials, syringes, ampoules, blow-fill-seal containers, IV bags and other pharmaceutical packaging use the latest generation of differential pressure testing or headspace analysis procedures allowing a highly sensitive and robust measurement.
Highlights: • Indus...
-
Product Formulation Development
Our Formulation Scientists and Process Engineers are experts in poorly soluble compounds and high containment operations. Our formulation development lab and pilot plant suites are specifically designed for high containment and outfitted with cGMP matching technologies. We follow a data driven approach to ...
-
Product R&D service
Neotron Pharma is able to offer you a research and development service. The research and development laboratory, operating under the ISO regime, will be able to develop customized methods for the customer in faster times and with lower costs. Subsequently, the customer will be able to validate the GMP meth...
-
Product Chemical Development Solutions
Chemical Development Solutions of GVK BIO provides a convenient, single-site distribution of infrastructure, thereby delivering a seamless and innovative transition of solutions from lab to pilot plant and bulk manufacturing, with speed and quality.
We ensure quality in the process during th...
-
Product Pharma Services / LDS
The Pharma Servicdes / Lichtenheldt development Department (LDS) accompanies and advises our customers from product idea through galenic development and through the registration into the routine production. For product development and scale up Lichtenheldt has its own dedicated staff and laboratories, ...
-
Product Analytical Testing and Formulation Development
Quality Chemical Laboratories is known for its capabilities in the development and validation of analytical methods for raw materials, drug substances, and drug products. With a strong focus on quality and precision, QCL employs cutting-edge technologies and a highly skilled team of scientists to offer com...
-
Product Biologic drug substance CDMO services
From pre-clinical development to commercial supply, Patheon by Thermo Fisher Scientific is an industry leader in the development and manufacture of mammalian cell culture drug substances. Patheon offers biotech and pharmaceutical companies the ability to pursue opportunities around the globe with a fully i...
Upcoming Events
-
CPHI Middle East 2024
Riyadh Front Exhibition & Convention Center Riyadh, Saudi Arabia
10 Dec 2024 - 12 Dec 2024 -
Pharmapack Europe 2025
Paris Expo, Porte de Versailles - Hall 7.2 | Paris, France
22 Jan 2025 - 23 Jan 2025 -
CPHI Japan 2025
East Halls 4, 5 & 6, Tokyo Big Sight, Tokyo, Japan
09 Apr 2025 – 11 Apr 2025
Pharmaceutical Industry Webinars
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance






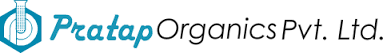
























.jpg)

.jpg)

.jpg)



































































.png)







.png)

.png)



.jpg)


