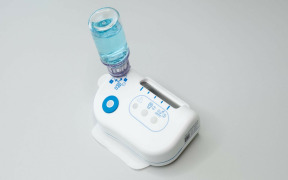
High-Dose/High-Volume subcutaneous (SC) drug delivery requires dedicated delivery technology to enable patient-centric and effective drug administration. The trend towards such high-volume delivery via the SC route is increasing and the Sonceboz OBI-Platform is the answer to many unresolved challenges in drug delivery such as proper feedback, tolerability, system flexibility. The Sonceboz OBI Platform is designed with three main requirements in mind:
1. Ease of use for patients and caregivers to prevent user errors through a combination of intuitive design and clear visual and audible messages. Furthermore, the devices are designed in such that users intuitively understand the mode of operation and preparation of the devices, proven by a number of human factors studies
2. Adaptation to Pharma’s preferred primary packaging such as vials, cartridges. Sonceboz does not impose proprietary containers or fill and finish processes. This reduces risk, cost and time to market. In addition, the LVI-V device can accommodate any volume from 1-20mL which provides a great deal of flexibility to Pharma when, for example considering use in clinical trial scenarios
3. Ease of integration into Pharma processes with the ability to stay outside the primary supply chain. By providing the possibility to store drug and device separately one greatly simplifies storage and supply related processes. Additionally, due to the frequent cold-storage requirements of Biologics, a separate drug and device logistics may reduce complexity and costly cold-chain storage space.
APPLICATION AREAS
High-Dose and High-Volume injection of parenteral drug formulations with a focus on:
- Immuno-Oncology
- Neuroscience
- Autoimmune disease
- Cardiovascular and metabolic disease
- Clinical Trials
- Lifecycle Management/transition from intravenous (IV) to subcutaneous
- Improvement of efficiency in hospital-borne treatments by simplifying subcutaneous delivery
KEY FEATURES
- Biologics compatible fluid-path
- Compatible with standard vials with 13 or 20mm neck sizes (glass and polymer)
- High-convenience compact footprint
- Full programmability of delivery (Speed, Timing, Pauses, Messages, Connectivity)
- Orientation independent dosing to ensure required dose is delivered
- Clear audible, haptic and visual feedback to prevent user errors and provide peace of mind
- Leverage on proven technologies derived from high volume manufacturing automotive applications