- Home
- Pharmaceutical Packaging
CPHI Online is the largest global marketplace in the pharma ingredients industry
-
Products551,744
-
Companies7,781
-
Articles11,636
-
Events8
-
Webinars342
Pharmaceutical Packaging
- Contract Services
- Drug Delivery Devices & Systems
- Injectable systems and components
- Medical Devices and Medical Packaging
- New/Smart Solutions
- Packaging Accessories
- Packaging machinery / production equi...
- Packaging Materials & Components
- Primary Packaging
- Printing / Labelling / Leaflets, Safe...
- Sustainable Packaging
- Tertiary packaging/Cool chain
- Testing, measurement, control, inspec...
Pharmaceutical Packaging Companies (166)
Pharmaceutical Packaging News
-
News Gerresheimer predicts weight-loss drug deals to account for 4% of yearly growth
Dietmar Siemssen, CEO of German primary packaging manufacturer Gerresheimer, states that approximately 4% of the company’s revenue growth each year to come from deals with drugmakers of weight loss and diabetes products, particularly GLP-1 class ...2 Apr 2024 -
News Novel approach to creating sustainable packaging from rice husks
Researchers have created a new approach to the designing of eco-friendly nanofibres extracted from rice husks, addressing the critical need for sustainable packaging materials in food and biopharmaceutical products.27 Mar 2024 -
News Unpacking what we learnt: Key Pharmapack takeaways with L.E.K. Consulting
After another successful Pharmapack show, we sat down with Karin von Kienlin, Senior Partner at L.E.K. Consulting, to discuss the biggest takeaways and surprises from 2 days of intense discussion and collaboration.14 Feb 2024 -
News Pharmapack Awards 2024 Packaging Innovation Award Winner – Nissha Europe GmbH
The 2024 Pharmapack Awards celebrated the best in innovation and design for the pharmaceutical packaging and drug delivery industyr on January 24, 2024.12 Feb 2024 -
News What did we learn from Pharmapack 2024? The green edition
Pharmapack Europe, held in Paris each year is a great opportunity to see the latest innovations and trends in pharmaceutical packaging and to hear from the companies and experts in the area on what aspects are most important to them in their products a...9 Feb 2024 -
News Pharmapack Awards 2024 Start-up Innovation Award Winner – Capa Valve Ltd
The 2024 Pharmapack Awards celebrated the best in innovation and design for the pharmaceutical packaging and drug delivery industry on January 24, 2024.2 Feb 2024 -
News Pharmapack Awards 2024 Patient-Centric Design Award Winner – Dr Ferrer BioPharma
The 2024 Pharmapack Awards celebrated the best in innovation and design for the pharmaceutical packaging and drug delivery industry on January 24, 2024.30 Jan 2024 -
News Women in Pharma: Minding the Gap at Pharmapack 2024
2024 marks the first year Pharmapack will host a Diversity track dedicated to bridging the gap within the pharmaceutical packaging and drug delivery sector. The track includes a panel discussion on 'Enabling Diversity in the Workplace,' focused...29 Jan 2024 -
News Pharmapack Awards 2024 - Celebrating Packaging and Drug Delivery Innovation
The 2024 Pharmapack Innovation Awards ceremony celebrated the best in pharmaceutical packaging and drug delivery innovation at all levels. The awards were held on January 24, 2024 at the Paris Expo Porte de Versailles.24 Jan 2024 -
News Pharmapack 2024 - From the Floor
Paris once again welcomes Europe’s leading trade show in pharmaceutical packaging and drug delivery innovation. Join our content team as Pharmapack 2024 opens its doors to leading experts and innovators in pharmaceutical packaging and drug delive...23 Jan 2024 -
News A Day in the Life of a packaging Project Engineer
To get to know the people working day to day behind our pharma companies, the ones who keep the wheels turning and ultimately bring better healthcare to the population, we would like to present a new interview series introducing pharma's talen...11 Jan 2024 -
News 2024 Pharma Industry Trends Outlook: Collaboration, Market Maturity, and Digital Futures
The annual CPHI Online 2024 Pharma Trends Outlook, in partnership with Arvato Systems, identifies 12 key industry trends shaping the life sciences industry in the coming year.9 Jan 2024 -
News On track at Pharmapack 2024 - The Track Sponsor interview: Gerresheimer
January 2024 brings both a new year and Europe’s leading packaging and drug delivery event. Gathering the world’s experts in pharmaceutical packaging together in Paris, France, Pharmapack 2024 offers exciting opportunities to learn and coll...2 Jan 2024 -
News CPHI North America 2023: from the experts – Sharp Services
Throughout CPHI North America (Philadelphia, PA; April 25–27, 2023), we caught up with some of the exhibiting organisations to ask what the show brings to the North American pharmaceutical sector, and what is driving industry innovation in this r...28 Jun 2023 -
News CPHI Online Webinar Series – The CPO Perspective: Driving Healthcare Sustainability
Pharmaceutical contract packaging organisations (CPOs) offer organisations broader expertise in pharma packaging, specialist skills, and flexibility with time, efficiency, and costings for pharmaceutical companies lacking in-house packaging expert...1 Jun 2023 -
News The CPO Starter Pack: Why and When to Bring in a Contract Packaging Partner
Discover the trends affecting the pharmaceutical CPO market, when and why outsourcing may be an enticing option, and what is driving pharmaceutical companies and CDMOs to partner with outsourced packaging and drug delivery organisations. ...18 May 2023 -
News Pharma Supply Chain People Moves
The latest appointments, promotions, and structural changes across the pharmaceutical supply chain.17 Apr 2023 -
Sponsored Content CPHI Podcast Series: Ursatec – celebrating 30 years of pioneering preservative free
In the latest episode of the CPHI Podcast Series, Digital Editor Lucy Chard spoke with Dominik Rocchi of Ursatec.17 Apr 2023 -
News Fully biodegradable nutraceutical packaging an industry first
An agreement signed between TricorBraun and BioBottles will give nutraceutical companies (and potentially pharmaceutical companies) in the US and Canada access to the industry’s first fully biodegradable packaging option.9 Mar 2023 -
News CPHI Webinar Series – The Big Questions: Pharmaceutical Packaging, Sustainability and Circularity
With growing awareness for sustainable industry practices within pharmaceuticals, pharma packaging companies are facing pressures to balance drug efficacy, patient safety, supply chain security, and sustainability. Speakers Sriman Banerjee, Head of Pac...17 Feb 2023
Pharmaceutical Packaging Products (500+)
-
Product Vision Robot Unit (VRU)
We present a modular, robotic, proof-of-concept system for inspecting parenterals. By employing artificial intelligence principles and applying the technology to the inspection task, we engineered the Vision Robot Unit (VRU).
The VRU is a reliable, autonomous island machine, capable o...
-
Product Pen Injector Assembly Line
Thanks to the modularity, we can provide tailor-made solutions designed to deliver high-quality standards even when scaling-up.Our Pen Injector Assembly Line is an example of a flexible mid-high speed pen injector line assembling different formats and integrating extensive in-process controls.
...
-
Product Primary packaging for biologics
Primary packaging for sensitive, biotechnologically manufactured active agents. Highest quality standards with regard to active agent-packaging interaction, increased user-friendliness and application safety for patients and user.

-
Product Customized solutions drug delivery systems
Customized drug delivery systems – from the concept development through industrialization to assembly, and pharmaceutical filling. We cover the entire spectrum of delivery types: inhaler, syringes, pen systems, auto injectors and infusion sets as well as lab disposables and point of care tests.
-
Product Commercial packaging services
Thermo Fisher Scientific offers a broad range of commercial packang services for clients across our worldwide network of commercial facilities in the EU, United Kingdom, and United States. Our broad range of capabilities can accommodate both specialty care and primary care products to ensure that your prod...
-
Product BlisBa - PVDC
PVDC coated films for blister applications
• for low to middle barrier demands • Duplex (DX) and Triplex (TX) structures
-
Product Analytical Services
Our state-of-the-art analytical testing labs support drug substance (API) and drug product (finished product) analytics across all phases of clinical development and into commercial release.
We employ over 170 highly skilled analysts working in GMP environments across the UK, Europe, and the...
-
Product Mikron MAIA - low volume assembly and test system to produce single or multiple variants
The Mikron MAIA is a low volume assembly and test system to produce single or multiple variants of medical devices such as auto-injectors, pen injectors or syringes. All key assembly processes are automated to ensure quality and data collection. The station parameters can be re-used for later productio...
-
Product MK™ Fine Mist Sprayer
Deliver precision dosing and superior spray performance for your Rx and OTC medications. Meet MK™—a fine mist sprayer designed to deliver precision dosing and superior spray performance. With its excellent priming, atomization, and clean output, it delivers an effortless spray experience for consumers. At...
-
Product CordenPharma Fully-Integrated Supply
CordenPharma’s vertically-integrated supply chain model provides development & manufacturing expertise spanning the complete GMP supply chain. Beginning with regulated Key Raw Materials and Non-GMP Intermediates, our sister organization WeylChem supports back integration to sec...
-
Product Child-resistant dropper set with Tamper-Evidence
Newly developed and childproof, the ‘CRC-Pipette set’ from Heinlein guarantees certified product quality and highest safety standards. Our CRC pipette is mainly suitable for liquids that place increased safety demands on the user. Its ISO 8317 and US 16 CFR § 1700.20 certification guarantees that these...
-
Product Flip-OFF-SEALS, SAFE DROPPERS, ADAPTERS, MEASURING CUPS, THERMOFORMING BLISTER COMPONENTS, SPATULAS, MASTERBATCH, OPHTHALMIC CONTAINERS, CANISTERS, ORAL SYRINGES, TYVEK PRINTING & INDUCTION SEALING WAD,
SAFE SEALS: DMF Grade Sizes : 13mm, 20mm, 28mm and 32mm
Colours : Can Match any colour per requirement
Finishes : Glossy and Matt. Custom Instruction Printing. Custom Logo Embossing.
Single...
-
Product Datwyler's components for Vial applications
Datwyler is the preferred solution partner to global pharmaceutical companies. We offer one of the most extensive product portfolios for vial applications in the pharmaceutical and biotech markets worldwid. Our range of products and services includes the most innovative elastomer formulations, coatings, al...
-
Product Complete lines for sachet and stick-packs in cartons
Universal Pack Synthesis complete automatic packaging Lines are individually custom-built, whether for primary or secondary packaging, guaranteeing the highest efficiency together with the most compact machine layout, perfectly in line with Universal Pack traditional concepts.
-
Product BOTTLES PE 28PP
Bottles in various sizes ranging from 50 to 300 ml. Accessories available.
For more information, please get in touch with us or visit our product catalogue: https://www.alplapharma.com/en/product-catalogue

-
Product Ophthalmic and lenscare: Tamper-proof dropper bottles
• Bottles with screw-on neck finish • Compatible with Röchling droppers & closures (3 part system) • Dropper Ø from 0,25 mm–1,2 mm (up to 2mm possible) • Tamper-proof closure, various colors available &...
-
Product Device Assemply and Packaging
The injectable drug market is rapidly evolving and includes an array of delivery devices from autoinjectors to pens and other intuitive formats. We provide partnership for drug owners driven by the top-quality, complex considerations and flexibility expected. These services include assembly process des...
-
Product Injectables (Aseptic Filling)
Specialist in filling and packaging SVP (small volume parenteral) in prefilled syringes and vials. Our injectable plants export over 50 countries worldwide.
- 5 high speed lines for syringes (aseptic filling)- 3 high speed lines for vials (aseptic filling)
- Terminal sterilization
- Fully equipped...
-
Product Rubber Components
Lonstroff, part of The Medical Rubber Division of Sumitomo Rubber Industries (SRI), is one of the world leaders in the development and manufacturing of innovative elastomers for injectable medicines. The Medical Rubber Division of SRI provides solutions for the pharmaceutical and medical industry, includin...
-
Product SELF ADHESIVE HANGING DEVICE
The Self Adhesive Hanging Device for infusion bottles is one of the latest Pilot Italia patents.
This innovative label provides big saving on the global infusion bottles packaging costs and gives to the product an innovative and hi tech appeal which is well appreciated from marketing division....
-
Product Extractables & Leachables Testing
Nelson Labs Europe offers a comprehensive approach toward Extractables & Leachables testing for the pharmaceutical industry. Our approach combines technical and analytical expertise, polymer knowledge and understanding of regulatory requirements, all combined in a tailored approach for our customers.
-
Product Analytical Services & EU Batch Certification
Method Development and validation
Stability Studies
European testing & batch certification

-
Product Primary Packaging for IV-solution bags
PolyCine offers:
Primary Packaging Films for IV-solution bag
Filling and transfer tubes for Primary Packaging
Hot Foils for IV-solution bag / primary container printing
Compounds for Primary Bag Connectors & Ports

-
Product Pharmaceutical Label Products
CCL Healthcare offers an extensive range of top-quality secondary packaging and labeling solutions. Developed by leading designers and engineers, our products lead the industry in quality and innovation. Tested thoroughly, available in various configurations, and customizable, we incorporate innovative fea...
-
Product Polyfoil® Mono-Material Barrier tube
Polyfoil® Mono-Material Barrier tubeEurope's FIRST fully approved Mono-Material-Barrier Tube
Cutting edge technology and research have led Neopac to their latest tube-industry innovation: the Polyfoil® Mono-Material Barrier tube (MMB). The pharma grade Polyfoil® MMB is a high-performance...
-
Product PHARMA PRIMARY PACKAGING | Combined product and manufacturing solutions
Rigid Cap Modern, multi-part sealing system for pre-filled syringes with intuitive screw-cap mechanism consisting of Rubber Cap, Rigid Cap and Luer-Lock-Adapter. Material: Steam-sterilizable >PC<, medical grade >PP<, biocompatible per ISO 10993-1 and USP Plastics Class VI
-
Product SENSOkidCAP®
SENSOPLAST® combines the advantages of tamper-evident closure with a child safety solution. The cap can only be removed from the bottle by simultaneously pressing and turning the cap. At the same time, tearing off the tamper-evident ring reveals the initial opening.
This special design has prove...
-
Product Drug Delivery Devices
Sanner Group develops and manufacturs your customized drug delivery device like inhalers or pre-filled syringes. Our pre-fillable syringes form a complete system when combined with the plunger rods and stoppers, the needle guard and finger rest enlargement.

-
Product BlisterJet 807
Digital blister printing system for late stage customization of blister production. Available with up to 2 spot colors. The BlisterJet is a fully digital piezo inkjet system. It prints on blank blisters. The solvent-free, UV-curable inks print sharp graphics and text in one to two colors. The system i...
-
Product Services - Lab analysis
We offer the quality standards that we apply to our own environment, machines, solutions and processes as services to our customers as well.
We make continuous investments in our specialist expertise. Quality assurance is the focus of our work. To ensure that we can meet your requirements r...
-
Product Foil for blister packaging
Symetal lacquered blister foils stand out for their compatibility to all different ink systems, while also offering excellent sealing performance against PS/PVC/PVDC substrates.
Accordingly, Symetal lacquered blister foil products serve as the perfect solution for pharmaceutical applications such as...
-
Product CLOSING TE
Bormioli Pharma offers a wide range of glass bottles for ophthalmic products which includes closing te. It belongs to ophthalmic dispenser category. Contact us for more information.
-
Product Aluminum Tubes
TUBE is equipped with the latest European technology to provide the finest quality, and the largest regional production capacity of aluminum tubes covering the diameters of 13.5 mm, 16 mm, 19 mm, 22 mm, 25 mm, 28 mm, 30 mm, 32 mm, 35mm, 38mm and 40 mm.

-
Product Labels
FEATURES - All printing technologies (Offset, Flexo, Screen and Gravure)
- Combination printing up to 14 colors
- Leading variety of digital printing options, incl. variable data printing for serialized fully...
-
Product Syringe accessories Green Line
RECYCLED MATERIAL? The material we use comes from the mechanical recycling of plastic bottles.
Once mechanically regrinded, the material is mixed with virgin material and extruded to form granules.
With this process, we are able to re-inject the material and manufacture the GREEN LINE.
...
-
Product Single-dose strips
A user-friendly and contamination-resistant alternative to traditional packaging methods.

-
Product EcoSecur type 2 glass
Stoelzle Pharma reached another milestone by developing a new, safe and resource-efficient process for the inner surface treatment of type 2 glass vials. This innovative technique enables reliable and precise dosing tailored to each bottle size, from the smallest 6 ml vials to much bigger. With EcoSecur in...-comp241444.jpg)
-
Product FEXUCLUE®
Fexuclue (Fexuprazan) is the best-in-class novel anti acid secretion agent with rapid onset time and potent acid suppressive effect, addressing the growing unmet needs of PPIs
Highlights of Fexuclue are: * Launched in Korea (2022) and Philippines (2023)
* Approved in Chile and Ecuador qq...
-
Product Vial & Syringe labels
Cinta-Plast provide a wide range of label solutions for vial identification.
We work with special white and clear films materials for vial identification.
Wide range of materials appropiate for a every temperature range.
Expertise in product serialization ( Datamatrix and QR)
Solutions for ...
-
Product Aluminium MDI Canisters (Primary Packaging for Metered Dose Inhalers)
Pressteck is a specialist for the production of deep-drawn MDI canisters made of stainless steel or aluminium. With our long-standing experience and our reputation for highest quality, we are a strategic partner to some of the leading pharmaceutical companies.Our canisters are used as primary packaging for...
-
Product DWK Life Sciences Primary Packaging Solutions
DWK Life Sciences is a leading global manufacturer and supplier of glass and plastic primary packaging. Our Primary Packaging container closure systems are manufactured from the highest quality pharmaceutical-grade tubing designed specifically for pharmaceutical, biotech, diagnostic/IVD, and personal ...
-
Product Production in class 7 clean room and Brickpacking
We offer an extensive range of PET bottles and jars that we can produce under Cleanroom conditions. Our Cleanroom production corresponds to Class 7 according to ISO 14644-1
-
Product VITALDOSE® PHARMACEUTICAL GRADE ETHYLENE VINYL ACETATE (EVA)
VitalDose® EVA copolymer is a technology platform for reliable sustained release performance in indications requiring long-acting drug delivery. Common applications of this ethylene vinyl acetate pharmaceutical grade copolymer include subcutaneous and ophthalmic implants and intravaginal inserts. Ther...
-
Product Nanopass™ 34G Pen Needle
Nanopass™ 34G Needle for Pen Injector features a 22% thinner double-tapered needle aiming at a better flow than comparable 32G designs.
-
Product Ophthalmic CDMO Services
Salvat can provide the best solutions, based on a long history of expertise in developing and manufacturing successful medications
• Tailored solutions to customer needs through its Ophthalmic platform. • Specific solutions using Salvat's technology: suspensions, solutions, micellar, and n...
-
Product Container Closure Integrity Tester for laboratory use
With our wide range of lab testing equipment we provide solutions for the early stages in the development processes as well as for quality control in commercial manufacturing. Being able to transfer the test method from R&D to production reduces risks and saves time.
The NEO series combines high...
-
Product Syntegon´s Solutions for the pharmaceutical industry
· Process technology
· Solid dosage forms
· Liquid dosage forms
· Inspection technology
· Secondary packaging...
-
Product Tamper Evident Solution
The Energy Saving Tamper Evident carton: without stickers and gluing.
SAVE MONEY: switch off sticker and gluing machines, run at the highest speed.
RESPECT THE PLANET: monomaterial, recyclable.
PROTECT PATIENT: the IGB seal is part of the box and needs to be ripped-off to open the box: it is IRREV...
-
Product Corning® 51-A Amber Borosilicate Glass Tubing
Corning sas offers a wide range of pharmaceutical Glass Tubes Tubes which includes corning® 51-a amber borosilicate glass tubing. Contact us for more information.
-
Product Contract Filling
With more than 50 systems in all sorts of configurations, Rommelag CMO runs one of the world’s most cutting-edge and largest bottelpack machine fleets. This allows us to perform quick and cost-optimized filling for you. Small-scale series, trial phases, or production batches? We’re ready for anything. And ...
-
Product Aidaptus
Aidaptus has been awarded the Red Dot award for the category Product Design 2023
Benefits
- Aidaptus® is a 2-step single use auto-injector platform, with a versatile design and intuitive drug delivery.
- Aidaptus® accommodates both 1mL and...
-
Product Medicine Spoons
Our products are manufactured in Class D Clean Rooms without human touch. Their colors are customizable. They contain no additives other than raw materials and dye. The raw materials, dyes, and the protective lubricants used to clean molds are food grade. The measuring lines on the product simpli...
-
Product Cold-forming Aluminum
Cold form blister packaging is especially useful for drugs that are sensitive to temperature, light, or moisture, as it helps to maintain the stability of the drug by providing an airtight seal. The blister packaging is also easy to open, making it convenient for patients to access their medicati...
-
Product Die-cut Lids (overall and zone-coated)
Oliver offers coated die-cut lidmaterials and custom design options for medical device and pharmaceutical packaging.Lids are fully customizable--including size, design and material selection--for optimal protection and performance.Lid materials include Tyvek®, Ovantex®, papers, films and foils.
-
Product Aseptic Fill and Finish Processing
Offering table top units for small batches up to customized high speed fully automated robotic production Filling and Capping Lines for syringes, cartridges, vials, bottles, ampoules, IV bags and various other containers.
• Cleaning • Sterilizing • Filling • Lyophilization • Closing •...
-
Product Folding Cartons
High functioning folding cartons offer an innovative, sustainable and cost-effective packaging format.
- Ability to apply Coding and Serialization - printed invisible, black light or IR inks or combinations
- Embossing including Braille to one or all panels.
- Tamper-evident and child-pr...
-
Product Rubber Punched Gaskets And Punched Plastic Gaskets
RUBBER PUNCHED GASKETS: With 70 years of experience in manufacturing punched parts, TekniPlex Consumer Products is a global market leader in aerosol industry applications. This experience makes us an ideal partner for manufacturers and co-packers who need a precision part, with tight tolerance for the...
-
Product Customized silicone syringe plungers by RAUMEDIC
Syringe plungers and stoppers made of our self-developed, slide-optimized silicone that comes in medical-grade quality have low break-loose force. This enables injections to be controlled and dosed much easier.
• Clean manufacturing conditions • Freedom of design and shape when using silicone • Small...
-
Product GREENIS® DISPENSER
This amazing dispenser is made of high content PCR PP resin and is RecyReady©.
Its attractive and intuitive way of applying cares presents a real advantage compared to many packages available on the market.
Designed as light as possible, using post-consumer resins or materials that are recyclable...
-
Product Shrink Tack Label - Light Protection
Our Shrink Tack Label now comes with a light protection function! Depending on your requirements, you can choose films from our substrate line-ups.
For example, the clear type allows the content to be visible, but your light sensitive drug is still protected from UV light, up to 380 nm of wav...
-
Product End-to-end contract manufacturing makes it simple
Only a true end-to-end partner can really simplify the entire manufacturing process. • One partner that works with you from product development and prototyping to full production.
• One partner with the metal and plastic expertise to make your complete device from the handle to the sharp “bus...
-
Product Folding Box
The best packaging is the one that combines accurate planning, effective design, the ability to serve the specific communication needs of each product and an impeccable technical realization for printing, folding and set-up. The production cycle uses 5 sheet-fed presses, 8 die-cutters and 10 folding/gluing...
-
Product The Blue Tube Evo
The Blue Tube Evo was the world’s first tube to be made of 100% recycled aluminium. And it closes the loop by incorporating aluminium packaging that has been used and thrown away by end-consumers. The Blue Tube Evo is the result of an innovative and future-focused eco-design approach. It offers a wealth of...
-
Product Full solutions provider
Nolato is a global full solutions provider and contract manufacturer specializing in product and process development through final device assembly and fulfillment. Our offering covers the entire value chain, from development to high-volume production and beyond.
Product development and design for ...
-
Product Continuous Thread Closures
• Compatible with standard SP-400 Finish HDPE Bottles & Capping Equipment
• Manufactured with US DMF approved raw materials and liners
• 100% automated quality checking with highly sophisticated Vision inspection systems

-
Product Machines for nested products
Multiformat machine for the filling and closing of nested syringes, vials and cartridges with liquids and semi-solids. A manual / semi-automatic unpack and loading unit for sterile packed "ready to use", nested containers and an automatic remove of Tyvedk foil and slip sheet is integrated. For viscous prod...
-
Product Clean room production for the life science market
We manufacture both individual components and fully-assembled applications. Using injection molding machines with a clamping force of up to 500 tonnes. From standard plastics or from high-grade technical thermoplastic, conforming to GMP Class C, ISO 7 or ISO 8. We meet clean room requirements as neces-sary...
-
Product Atec Transfer Port - ATP
The Atec Transfer Port acts as a closed transfer lock to enable sterile transfer of components into and out of isolators and RABS. A unique locking system was developed to guarantee both operator safety and product integrity. The ATP cannot be opened unless the Beta port is correctly docked. This also mean...
-
Product INVISTA medical polypropylene resins
MELITEK offer and distribute PP resins in Europe from INVISTA (former Flint Hills) USA, who has a wide and unique range of medical PP materials. INVISTA has for many years been leading supplier of medical PP in US market. We have proudly serviced the product range to European customers for over 30 year...
-
Product Plastic injection molds
Mold design and manufacturing for Medical and Diagnostic in Vitro plastic devices.
-
Product Development & manufacture - drug delivery devices
Bespak by Recipharm delivers market leading design, development, and manufacturing services for drug delivery devices to the global pharmaceutical market. Our portfolio includes inhalers, nasal technologies, and auto-injectors as well as development and manufacturing services.
-
Product ABC Transfer® Betacleanbags™
Safeguard patient’s health with our range of next generation bags.
ABC Transfer® Betacleanbags™ combine cGMP compliance, ultracleanliness, ergonomics and eco-responsibility.
Our range of next generation Single-Use Ported Bags bring significant improvements to your sterility a...
-
Product Vial Adapter Ø13 and 20 mm
A full range of Vial Adapters devices for drug transfer and reconstitution, in blister, with or without filter 5 or 15 micons.
Contact us for more information.
-
Product Injectable vials in molded glass - Clareo
Clareo, the highest quality Type II premium injection vials Clareo, the highest quality Type II premium injection vials Clareo vials are made of Type II glass and offer a combination of homogeneous wall thickness with superior cosmetic qualities suitable for high value products. Available fro...
-
Product Device Design & Development
From initial concept design to industrialization, Jabil Healthcare Engineering & Technology (JHET) + our wholly owned subsidiary Radius Innovation & Development help the most trusted healthcare brands to solve their increasingly complex product design and development chall...
-
Product Smart hearing health
TympaHealth, one of our start-up clients, launched the world’s first all-in-one hearing health assessment system, a portable smartphone-enabled otoscope with accompanying smartphone app. Since then, the novel device has been awarded Gold in the internationally recognised UX Design Awards for its innov...
-
Product Small embeddable RFID micro tag for Syringe, Vial, Cartridge Serialization
Product Brief
The RFID micro tag can be fit on or embedded into any metal/non-metal objects, such as rigid needle shield, and rubber plunger.
This product can be used globally with high performance and reliability.
Why is RFID necessary for Pre-f...
-
Product Compression Springs
Advanex produce a wide range of compression springs and from 0.1mm to 3mm wire diameter in a wide variety of materials from carbon spring steels to alloy wires. With full inhouse design capability, our Technical Department can help you find the optimum design for your device, ensuring your product is no bi...
-
Product Grid coated paper
Grid coated paper offers optimised heat sealing characteristics and can provide low, medium or high seal strength. It is available unprinted or printed with up to 6 colours.
Our modular concept for grid coated paper includes:
• medical grade paper: 60 / 80 / 100 g/m²
...
-
Product ESD-Staticare®
Antistatic Cleanroom Packaging to ensure perfect protection of products
Characteristics:
-ESD - permanent antistatic
-flexible LDPE-film
-Cleanroom Production: GMP Class B
Staticare® - migratory - antistatic properties -...
-
Product NOTIPLUS®
Our offer: an all-in-one packaging, bottle, case, instructions, NOTIPLUS®.
All-in-one packaging with integrated leaflet to optimize packaging design and reduce the carbon footprint of products A multi-format leaflet, up to 35 pagesA mono-material label, integrating 30% certified PCR
...
-
Product High Speed Insulin Injection Pen Assembly Line
Customized High speed indexed assembly machine with 100% in line control
-
Product Multipage Labels
The original self-adhesive multipage label provides additional space to convey methods and instructions for use, warnings, legislative references, product range and manufacturer’s information.
Adaptable to any type of package, flat or cylindrical, it is ideal for providing multilingual ...
-
Product Injection / Thermoforming Contract manufacturing
Injection / Thermoforming Contract manufacturing
-
Product PEKUWhite - Cleanroom packaging - PE bags / films / tubes
PEKU Folien - Cleanroom and Pharmaceutical. Your Packaging. Our Passion. Film extrusion, Converting, Co-Packing according ISO 9001 and DIN ISO EN 14644 grade ISO 7 / EU GMP -B in operation, ISO 5/EU GMP- 5 at rest;
PEKUWhite: Tublar film, center folded film, flat film, bottom seam bag, ...
-
Product DROPPER BOTTLES
For Special Cap and Pipette
For D30 Cap and Pipette
http://www.neutroplast.com/en/products/dropper-bottles/
-
Product Pharmaceutical leaflets
Intrograf has a separate production area dedicated to leaflet production with sustained conditions and continuous control of such parameters as humidity and temperature. This allows us to optimize production and to print and fold low grammage paper leaflets of various sizes and folding styles.
-
Product ClearVial - COC or COP vials
MEDICOPACK A/S provides a wide range of vials which includes clearvial - manaufactured from COC (COP only available upon special demand). It is transparent, lightweight and with good impact strength, clearvial is a breakthrough in primary packaging solutions for the pharmaceutical and biotech industries. M...
-
Product Secondary Pharmaceutical Packaging
Secondary Pharmaceutical Packaging
Cartoning Machines
Clocked horizontal cartoning machines for folding boxes, also with 5th flap Solo or inline systems
Leaflet folding for package inserts, max.: 210 x 600 mm
Coding, code reader, data matrix code (with / without serial number...
-
Product GasSpect O2
The Gasporox sensor series GasSpect offers non-destructive Headspace Gas Analysis to be integrated into an inspection machine. The sensors with its highly sensitive laser impresses with performance and precision. The GasSpect series comes in different variations depending on which gas to be inspect...
-
Product INTRAOCULAR LENS INJECTORS
Ophtamologic injector with a body, a pusher, a metal spring and a sleeve (plunger).
-
Product ACLAR ACCEL™
WHEN “GOOD ENOUGH” IS NOT ENOUGH, ACLAR ACCEL IS THE PERFECT GO-TO SOLUTION.
Joining our flagship line of Aclar films, Honeywell Aclar Accel is a high moisture barrier thermoformable film product that delivers at the price and speed business demands while providing the quality and servic...
-
Product Applicators
Elm-plastic GmbH offers a wide range of products which includes applicators. Features: it is used for oral application of vet.-med. Pastes and gels. The ergonomic shape of the elm-plastic applicators guarantees comfortable and secure handling. The special geometry of the piston ensures an unproblematic fil...
-
Product Package inserts
As a recognised specialist in package inserts, we are the perfect partner for virtually any package insert project. From simple, monochrome inserts to outserts and double or triple leaflets with single-colour or multicoloured print, we produce and fold your chosen products to create the sophisticated combi...
-
Product Closure 1.4k (DIN18)
Closure 1.4k is one-piece screw closure, which guarantees reliable product protection against counterfeiting and accidental tampering.One-piece covers with a protective ring are easy to use and provide tightness, as well as strong fixation.
It can be equipped with various droppers.
Pharm...
-
Product RayDyLyo®
RayDyLyo® is a new standard for vial capping. Alternative solution to aluminum crimped cap, RayDyLyo® is a patented range of plastic press-fit closures, solving long-standing issues in aseptic production from the capping process and from cosmetic defects. RayDyLyo® is a breakthrough innovation, whi...
-
Product Assembly and test systems for medical disposables / plastic assemblies
Dispensing pumps, stopcocks, tubing sets and other medical disposables are mass-produced and marketed in huge volumes. We have developed the fast-cycle TEAMED RTS assembly system especially for this purpose. Its mechanical cam drive guarantees stable processes and high volumes for decades to come - 2...
-
Product anti-counterfeiting features // ORIGA
Lidding foils with this feature are destroyed upon opening and cannot be restored. This enables us to guarantee that the medication is in its original state.
- Tamper-evident feature is activated when the lid is peeled off the thermoformed tray
- This feature developed by Constantia Fl...
-
Product Medical devices for gynecological application
INGE produces medical devices for vaginal liquid products, creams and suppositories. 50 ml to 180 ml bottles for vaginal shower made of a soft, easily compressible material, also Green. Bottles are available in various shapes - cylindrical or elliptical - customizable with front and back engraving and one-...
-
Product ASM 2000
Helium Mass Spectrometry
Tracer gas leak detection and especially Helium leak detection is still the most sensitive commercially used test method on the market. Helium leak detection is only method able to detect sub-micron defects (far below the sterility level), instantly without storag...
-
Product Cleanroom plastics injection moulding by GEMÜ
GEMÜ produces an extensive range of plastic injection moulded parts. The market segments we serve, such as drug delivery, medical devices, diagnostics, or consumables, require a production environment with the highest cleanroom standards as well as the ability to work with varying part sizes and indivi...
-
Product Syringes packaging solutions
Products advantages : • Customized solutions to fit yours needs
• Secure closure, No touch packaging ...
• Sterilizable : gamma or bêta rays, steam
Our standard range includes no touch blister in PETG, syringes boxes, folding blister, multisyringes shell PC, customized velbox...
-
Product Pentapharm® BlisterPro® XCEL Services
Imagine design and prototyping Services that enable accelerated market introduction with increased accuracy in stability testing using the most cost-effective blister film to meet your requirements. Contact us for more information.
-
Product Healthcare Polymer Solutions
Our wide-ranging product portfolio includes brands by well-known polymer manufacturers. We are therefore able to provide a wide variety of different plastics that have been specially developed for the healthcare sector.
-
Product Contract Assembly and Packing
We can support you in:
- Automated and Manual Product Assembly & Kitting
- Blister Filling and Sealing
- Pouch Filling and Sealing
- Form Fill Seal Packaging
- Labelling
- Secondary & Tertiary Packaging
- (Pha...
-
Product plastic primary packaging
closures and containers for pharmacy, CRC and TE caps, push on TE caps, containers with screw on and push on thread
-
Product CombiSys
// Flexibility meets zero reject principle. ///
CombiSys is an efficient and highly productive system solution that enables you to process various packaging materials on a single platform. The transport system uses grippers that give you a wide working range without changing size parts. Modul...
-
Product GSS P Steam Sterilizer for pharmaceutical production
GSS P is a steam sterilizer from Getinge for component sterilization in pharmaceutical production. GSS P ensure a reproducible process, product quality and a safe environment. This makes it easier to achieve high performance, productivity and a streamlined process.
Getinge develops, manufactures a...
-
Product Plastic bottles and closures
Drug Plastics has been designing and manufacturing HDPE, LDPE, PP and PET bottles and closures for over 50 years. Our products offer the highest level of material control and process integrity; and we manufacture all of our pill bottles, plastic liquid bottles, and complementary closures with relentle...
-
Product Tyvek®
Tyvek® coated or uncoated, is a breathable material made with pure polyethylene fibers, which guarantees an optimal protection from microbial penetration, against tearing and perforation.
Tyvek® is characterized by an excellent peel ability during opening a medical device, if welded with peela...
-
Product Cartoning Machine MA200 - 202
Continuous motion, high speed, horizontal Cartoning machine, suitable for packing products into three-flaps straight or reverse tuck-in cartons.
Main features:
- Design, manufacturing and ergonomics, in accordance with cGMP norms- Versatility and efficiency in a small footprintBalcony d...
-
Product Divider in solid PP
Interlocking divider obtained from solid PP sheet, die-cut and assembled.
-
Product Thermoform Packaging Machine: RX 4.0
Innovation at its best !
The RX 4.0 offers maximum process reliability. Using closed control circuits, the sensor system constantly captures all the relevant process parameters, such as those for forming, evacuation and sealing. All the process stages are coordinated to the optimum level...
-
Product ALUMINIUM PILFER PROOF CAPS
suitable for pilfer proof bottle mouth, and available in diameters from 17 mm up to 38 mm
different colours, different logos different liners or insert (like dessicant)

-
Product SERIALIZATION
We offer total expertise for the serialization of your individual products. For that we use the most modern technologies available for printing, packaging and labelling solutions. Our intelligent software systems ensure a smooth serialization throughout the manufacturing process, which guarantees...
-
Product Omag Packaging Machine mod. CP
Intermmitent motion packaging machine with sealing plates technology to guarantee perfect sealing and high quality of the sachets, without any wrinkles in order to maintain the integrity of the product. Possibility to pack different products in classic 4 side sealed sachets or shaped sachets. The idea...
-
Product Primary packaging
• PVC // Alu • PVC/PVDC // Au • PVC/PE/PVDC // Alu • Alu // Alu • controlled substances packaging • packaging of potent OSDs • bottles (glass, plastic, aluminium) with different caps (push-on, screw, snap safe, desiccant capsules) • unit dose blister
-
Product Jekson Vision Product Portfolio
JEKSON VISION is a global provider of vision inspection and track & trace solutions for the pharmaceutical industry. By innovating machine-vision for industrial automatic inspections and traceability, we offer our customers new solutions from our 20+ years experience.
Our key focus is on res...
-
Product ENGINE ASSEMBLY AND CONTROL PLATFORM
This platform allows the assembling of cosmetic engines with in process controls .
-
Product Delta Printing Machine
This machine is simple to set up and changeover. The secret is the 109 snap-in carrier links. It can easily be installed or removed in 3 minutes without the use of tools. Full set up with design rolls, ink and rubber rolls can be accomplished within 30 minutes. Its features includes : a dual chain, interlo...
-
Product Cap + spatula / brush
SOPAC MEDICAL offers a wide range of services which includes cap + spatula / brush . Contact us for more information.
-
Product CT1
Sarong S.p.A. offers a wide range of machines which includes ct1. Features: it is used to connect the machines to an alternate motion cartoner, complete with the stainless steel buffer container to contain the suppository band. The a.m. Group can cut the suppository band in strips of 3-4-5-6 suppositories ...
-
Product PLASTIC BOTTLES WITH TAMPER-EVIDENT BAND
Plastic bottles (plastic containers) with tamper-evident band for pharmaceutical use are used for packing (storing) liquid pharmaceutical products (magistral medicine, galenical medicine, ready-made medicines, and pharmaceutical substance). They can be in direct contact with the product. These containers a...
-
Product Applicator
What Makes An Applicator Interesting? • Controlled, precise application of product • Accurate control of the product flow by choosing the spring tension, the opening hole and the cover materialSuitability for a variety of applications, e.g. • Acne therapy, insect repellent, sunscreen, &...
-
Product Injection vials
Our injection vials are mostly used for human and veterinary medicine, analytics and laboratory supply. Please find some more information including the standard sizes on our website: https://pharmaglas.de/injekten.html
-
Product Gamma Irradiation
There is much to consider when choosing a sterilization technology. Sterigenics’ expert advisors look forward to working with you to understand and achieve your unique product goals. We offer all major sterilization technologies: Gamma Irradiation, Ethylene Oxide & Electron Beam as well as innovativ...
-
Product Oral Syrups - Suspension/Dry/Liquid
Pharma Access is an end to end Solution provider for timely execution of Complete Turnkey Projects in the field of Pharmaceuticals, Vaccines and Biotechnology in all pharmaceutical forms viz-a-viz;Inhalation (MDIs and DPIs) Sterile injectable (SVP, MVP and LVP) and non-injectables Oral Soli...
-
Product Airless Motion®
Gaplast provides packaging and applications of plastic, for the pharmaceutical, cosmetics and medical applications which includes `Airless Motion®. It belongs to Airless Motion® products category. It is pumps with snap-on, crimp and threaded neck. Applications: liquid and semi-solid products, pharmacy,...
-
Product Soliboard folding cartons
With more than 2,5 billion folding cartons produced each year for the Pharmaceuticals / Personal care sector, LGR Packaging has a perfect knowledge of the product and offers a wide range of solutions, regarding design, printing, finishing, functionalities.
• DESIGN: simple co...
-
Product Veterinary Syringe
Xinfuda specializes in the production of standard and customized veterinary syringes. 15ml 20ml 30ml 60ml syringes with wide nozzle are suitable for the oral of gels, creams and vitamin pastes to horses, sheep and pets. Our paste syringes consist of four components: barrel, cap, plunger and dosin...
-
Product Folding Boxes
• Folded cardboard boxes with gluing in 1, 2, and 3 points • Straight and folded leaflets Raw material:
• GC, GD, GT cardboard of weight between 230 and 450 g/sqm • Offset paper of 40, 45, 60 g/sqm
-
Product Drug Product & Finished Goods
End-to-endservices designedfor patient safetyand centricity while offering contracting convenience, cos tefficiencies and time savings. Our globally integrated drugproduct (DP) and finished goods (FG) services offer agileand flexible solutions for clinical to commercial products. Our DP capabilities includ...
-
Product Oral, Vaginal, Rectal
We offer millimeter solutions in medicine applicators and dispensers, being today a global reference for quality and precision in this segment.
Our systems aim to be used for oral, vaginal and rectal treatments such as Oral infections, Vaginal infections, and Rectal diseases.

-
Product Wide neck drums
Wide neck drums have been designed to provide the ultimate protection for your valuable products. Its signature screw top closure provides protection against moisture and is safe, fast and easy to operate.
Plastic High Performance Packaging by CurTec is clean, safe, and secure and offers maximum...
-
Product Aerosol Cans
A new generation of innovative, alternative-shaped aluminium aerosol cans are already a new standard which fits the individual needs of our customers. Rimmed, shaped, transfer flat shoulder, bossed cans are all possible for Perfektup Aerosol Business Unit to produce. Perfektup Aerosol has been producing ri...
-
Product Pharmaceutical Packagings
Combi Seal
Euro Cap
Flip Off
LDPE,Pharma Grade
Moulded Glass Injection Vial, Type I/Ii/Iii
Rubber Stopper
Plunger
Prefilled Syringe
PP,Pharma Grade

-
Product HDPE Bottles
We provide an unmatched combination of product offerings & packages, design & innovation capabilities to leverage new technology & patient insights, & manufacturing expertise in pharmaceutical packaging
To make Berry a part of your Healthcare manufacturing process, plea...
-
Product Aseptic Processing / Syringes with solid implants
Harro Höfliger Verpackungsmachinen provides wide range of products which includes aseptic processing / syringes with solid implants. It is to equip a syringe with the solid implant, the syringe barrel is disassembled. After completion of the product, it is labeled and subsequently fitted with a protective...-comp272211.jpg)
-
Product 3K®-SYSTEM
The non-airless dosage system for dispensing liquid pharmaceutical or cosmetic formulations
3K = triple protection against microbiological contamination: • Bacteriostatic surfaces • Microbiological tight closing valve • Air purifying filter Both a microbiologically tightly closing valve&n...
-
Product C80HS - Blister packaging machine with integrated cartoner
The line’s innovative design, in terms of both structure and functional detail, has been developed keeping in mind the market request. C80HS Series, double-lane blister machine, not only has high production but also has the advantage of being extremely efficient: even if the downstream machine stops the re...
-
Product All-electric IntElect machine
Our all-electric, high-precision injection moulding machines from the IntElect series with acid and clean-room capability are the perfect fit for manufacturing products in diagnostics, medical and laboratory technology.Thanks to its design features, the all-electric IntElect injection moulding machine is a...
-
Product Rubber Stoppers for Antibiotics
Sanok Rubber Company S.A. offers wide range of products which includes rubber stoppers for antibiotics. Contact us for more information.
-
Product GL 18
Kunststoffwerk Kremsmunster Gmbh provides wide range of closures which includes GL 18. Executions: closures without tamper evidence ring; closures with tamper evidence ring; child resistant closures; closures with hole for pipettes; flip-top closures. Functionality: sealing feature; in combination with sta...
-
Product Collapsible aluminium tubes
Collapsible aluminium tubes are available from Ø13,5mm-40mm.A broad choice of closures and printing up to 6 colors ensure the appreciated customization of the product.
Following the GMP guidelines, the respect of the requirements in the pharmaceutical and cosmetic segment is ensured.
-
Product 20ml Screw-on Nasal Spray Bottle for Decongestant
BONA Pharma offers a wide range of products which includes 20ml screw-on nasal spray bottle. It belongs to spray bottles category.application: it is suitable for glass vials screw-on solution: best suited for the pharma, healthcare markets. Dispenser sub type: bottle - sprayer/pump. Contact us for more inf...
-
Product Pentapharm® kpSilverTM films
Klöckner Pentaplast introduces Pentapharm® kpSilverTM films designed as a highspeed thermoforming option with an aluminum look comparable to cold-formfoil packaging. The metallic look of kpSilverTM provides the visual characteristicsof cold-form foil while providing outstanding quality with significant pro...
-
Product Polypropylene Homopolymer
Borealis AG offers polypropylene homopolymer. It is used for wide applications in pharmaceutical industry. Contact us for more information.
-
Product Serum vials
Sio2 medical products. Provide wide range of pharmaceuticals which includes serum vials. Contact us for more information.
-
Product HIPACK COLD-FORM ALUMINUM FOILS
Cold-form high-barrier composite films foils for pharmaceutical blister packaging.
-
Product Intramammary/Paste syringes
Hubart De Backer offers a wide range of products which includes intramammary/paste syringes. Variable nozzle opening, one-piece plunger. Contact us ([email protected]) for more information.
-
Product Activ-Polymer Technology
CSP Technologies offers a wide range of products which includes activ-polymertm technology. Features: it has an active particle that provides the desired characteristics, such as absorption or adsorption of moisture, gasses or odors; release of aromas or antimicrobials; or transmittal to enhance the gas tr...
-
Product Anti-counterfeit labels
Solutions to protect your products against fraud and counterfeiting.
-
Product Epidural cannula
Sfm medical devices gmbh offers wide range of products which includes epidural cannula. It belongs to neurology / regional anesthesia / pain therapy category. It has developed a stable and functional epidural cannula for use in regional anesthesia. Special attention to the haptics of the neck and wing incr...
-
Product Flexi-Cap
Schreiner MediPharm offers a wide range of product solutions which includes flexi-cap. Solutions: It has developed a security solution that clearly and irreversibly shows a primary container has been opened. Therefore, the label is combined with a film cap, similar to the cover for wine bottles, but which ...
-
Product Composite sheet
Sichuan Hui Li Industry Co. Ltd. offers a wide range of composite sheet for packaging which includes composite sheet. Contact us for more information.
-
Product Filter plates
Pöppelmann GmbH & Co. KG offers a wide range of laboratory equipments & dignostic products which includes filter plates. Contact us for more information.
-
Product Bottles
Bharat rubbers works offers a wide range of packaging products which includes Bottles. It manufactures plastic dropper bottles for eye and ear drops. Clean room technique is applied to ensure product is dust free. Eto sterilization process is also used to ensure that product is free of micro organisms left...
-
Product Analytical and Testing Services
With our Technology Excellence Centers (TEC) we can offer analytical and device testing services to support you all the way, from early-stage to launched combination product.
2 locations, 1 goal: assisting customers to anticipate challenges and help you navigate the regulatory landscape.
q...
-
Product AquaBa - Super B
Super B PVDC coated films for blister applications • for high to ultra high WVTR & OTR barrier demands • Triplex (TX) and Sandwich (SW, symmetrical) structures
-
Product Aclar® - PCTFE
PCTFE laminated films for blister applications • for high to ultra high WVTR barrier demands • Duplex (DX), Triplex (TX) and Sandwich (SW, symmetrical) structures
• Aclar® is a registered trademark of Honeywell International Inc.
-
Product BlisForm - PVC (Mono)
PVC Mono-film for blister applications • for low barrier demands • standard to high deep draw abilities
-
Product BlisForm MA - APET
A-PET film for one-material blister applications for low barrier demands, standard - high deep draw abilities.
-
Product BlisLid MA - APET
PET lidding film for one-material blister applications for low barrier demands.
-
Product BlisForm MP - PP
PP film for one-material blister applications for low to middle barrier demands.
-
Product BlisLid MP - PP
PP lidding film for one-material blister applications for low to middle barrier demands.
-
Product AluBa - ColdForm
Coldform Tri-laminates for blister applications for ultra high barrier demands, improved formability due to primered aluminium.
-
Product Liveo Research Services
Our service portfolio contains:
Optima - Packaging Solutions that pay off.
FastPack - Faster speed to market.
Modelling - Cost effective shapes without any compromises.
Lab - Your choice for analytical services.Liveo
EZ Switch - We can make switching easy.

-
Product CordenPharma Pharma Packaging & Logistics
• PACKAGING DEVELOPMENT - CordenPharma manages all the logistical procedures involved in packaging development by creating and producing your packaging components. From layout development and producing a printable copy to procuring the materials, we work with creativity and precision. • PACKAGING -&...
-
Product Mark VII® Max
Smooth, controlled dispensing for a wide array of dermatology products
Combining excellent priming with smooth actuation, Mark VII® Max gives your customers what they want—consistency and control. It is compatible with high-viscosity and difficult dermatological formulations, making it ideal for s...
-
Product M3
.A travel-size evolution of our F3 and G3 handheld pumps, M3 gives consumers the convenience of portable dispensing for their skincare, personal care, sun care, hair care, and kids’ care products. Its rounded shape provides a foundation for customization, from nozzle design to foam characteristics. Add eff...
-
Product Silica Gel ,Activated carbon, Molecular Sieve , 2 in 1 (Silicagel + Activated carbon) & Oral Syringes and HDPE Canisters
Materials used in manufacturing of sachets like Tyvek, Silica Gel, Activated Carbon, Molecular Sieve and inks are food graded and meant for pharmaceutical packaging.
Material used in manufacturing of canisters like HDPE Can, Lid, Silica Gel and labels are food graded and meant for pharmaceuti...
-
Product Pharmaceutical Development - Late Phase
Enabling Confidence and Security in the Supply Chain
Specialising in solid oral dosage forms, Almac has the technical experience and knowledge to develop robust formulations and manufacturing processes.
We have assisted many of our clients in the development of both immediate and mo...
-
Product Datwyler's solutions for Prefilled Syringe applications
We offer our customers a broad portfolio of the highest quality sealing solutions for their syringe systems. This includes elastomeric plunger stoppers, needle shields, and tip caps to seal the prefilled syringes as well as plungers and rubber-lined aluminum seals to ensure system integrity.
-
Product Datwyler's wide range of Cartridge components
As a reliable supplier of sealing solutions for sensitive drugs, we offer an extensive product portfolio in cartridge applications. With this broad offering, we can make an important contribution to a variety of pharmaceutical and biotech therapies, such as dental care and insulin management. Our priority ...
-
Product Mikron Polyfeed - standalone flexible feeding system
You can count on it for unmatched flexibility, short delivery time, reusability and speed. Mikron Polyfeed is a flexible feeding system based on the use of visual recognition and vertical vibration systems to identify parts in their various forms and positions. The feeding speed can reach up to 100 pic...
-
Product Mikron G05 - modular assembly and test cell
The Mikron G05™ linear assembly cell is based on the use of standardized building blocks which offer flexibility and modularity. The proven and stable Mikron G05 platform ensures speed, precision and reliability of your automation solution.
The platform makes use of a cam drive for all p...
-
Product Mikron manual bench - for medical device assembly
The system allows customers to test the design on a small scale to ensure processes are correct and match the device assembly specifications. The manual bench is used primarily for pen injectors, auto-injectors and safety syringes.

-
Product Mikron Tray Handler - highly configurable handler
Mikron supplies a highly standardized tray handler capable of dealing with a range of applications from single tray to multiple stack, for tray sizes from 300 x 400 to 400 x 600 mm, and designed for parts that are susceptible to damage or contamination if fed using other methods such as bowl feeders. q...
-
Product HDPE Bottles, PET Bottles, PP Bottles, HDPE Bulk Drums, PP Closures(CRC Patented), Inner and Outer Cartons, Inserts, Aluminium Foil for Printing & Leaflets
Flexibility of manufacturing containers on EBM or IBM (Auto De-Flashing System) with 100% Automated Inspection.
We are committed to manufacture the packaging materials of right quality at right time to the customer satisfaction.
We endeavour to continually improve the quality management...
-
Product EMPTY HARD-SHELL-GELATIN-CAPSULES
All our capsule variants are suitable to hold medical formulations in the form of powder, granule, semi-solid blend, pellet, etc. These empty capsules can also be printed with your brand name or logo to add authentication to the product. We maintain our product & production facility as per requirements...
-
Product Round nasal spray bottles with snap-on finish 20 mm
• Full range from 10 ml to 50 ml • Also suitable for crimp-on pumps • Delivered ready-to-fill (sterile) or gamma sterilized • Officially approved by Aptar Pharma, Nemera and Silgan
-
Product PET injection bottles with crimp neck finish
• sterilized available • available in 20-500 ml • other sizes and colour on request
-
Product Pharmaceutical Product Launch and Distribution
Pharmaceutical Product Launch and Distribution
Having successfully partnered in the launch of 15 significant orphan and niche drug products into the EU market over the last 3 years, Almac is the industry leader for Pharmaceutical Product Launch and Distribution.
q...
-
Product Commercial Drug Product Manufacture
Over 40 years’ Experience Manufacturing Solid, Oral Dosage Forms
Scaling up from our development services to full scale commercial drug product manufacture or transferring existing commercial products we can meet your commercial drug product manufacturing requirements.
Our range of com...
-
Product Serialisation
Serialisation and Track & Trace
Addressing evolving global serialisation legislation, Almac’s expert multi-disciplinary serialisation team lead the way in supporting clients in developing their serialisation strategies to GS1 standards.
...
-
Product Commercial Storage and Distribution
Complementing our commercial manufacturing and packaging services, we provide a range of commercial storage and distribution solutions for solid oral dosage forms and sterile biopharmaceuticals, for both niche / orphan drug products and large scale commercial market volumes.
Utilising our bespok...
-
Product Pharmaceutical Development - Early Phase
Almac’s experienced formulation scientists can develop a range of oral dose formulations for early stage clinical trials.
With both non-GMP and GMP facilities, flexible and efficient solutions are provided to develop a fit-for-purpose formulation for your early phase clinical trial.
...
-
Product Solid forms Manufacturing
Our solid forms plant exports 75% of its output, supplying products to 45markets in 25 different languages.One of the biggest FDA-approved plant for solid forms in Europe with an annual capacity of 3 billion tablets. We use the latest technology to produce high-quality products ata very competitive Price.L...
-
Product Water for injections
Rovi offers Water For Injection (WFI) in prefilled syringes in different sizesand volumes, produced according to European Pharmacopoeia (Ph. Eur.) and United States Pharmacopeia (USP) requirements.Module 3 common technical document (CTD) dossiers and drugmaster files (DMF) are available toour WFI clients a...
Pharmaceutical Packaging
Packaging is an essential part of every manufacturing and production process. Pharmaceutical packaging involves the provision of an appealing presentation, portable containment, reliable protection, convenience, and identification information for a product for storage, distribution, and sales. This containment is essential for preserving drugs and drug products during storage, transportation, and display, till the drug or drug product is consumed or administered.
Pharmaceutical packaging must be designed in such a way that it protects the drug or drug products from external factors such as temperature, sunlight, micro-organisms, etc., and maintains its stability. In the healthcare sector and the pharmaceutical industry, pharma packaging plays a significant role in the safe procurement and administration of pharmaceutical products. Here, we have carefully examined the types, categories, the market size of pharmaceutical packaging, and its importance to the pharmaceutical industry.
Categories of pharmaceutical packaging
In the healthcare and pharmaceutical sectors, close attention is given to pharmaceutical packaging as it is used to complete the medical prescription of various drugs for identification, storage, and consumption. There are three categories of pharmaceutical packaging used for every pharmaceutical product regardless of the dosage form. These categories include primary, secondary, and tertiary packaging.
Primary packaging consists of materials that have direct contact with the drug or pharmaceutical product packaged. This is the material surrounding the drug. The materials must be inert, safe, and capable of protecting the product from external factors such as moisture, climatic changes, biological contaminations, etc. Some primary pharmaceutical packaging types include sachet packaging, bottles, glass vial, injectables, blister pack, ampoules, etc.
Secondary packaging refers to boxes or cartons where the package is kept. This usually contains information and directions of the pharmaceutical product. Tertiary packaging refers to the last stage or level of packaging carried out to aid transportation, shipping, and wholesale storage. Tertiary packaging primarily protects the product, primary and secondary packaging from external factors during transit.
What are the types of pharmaceutical dosage form containers?
There are different types of pharmaceutical dosage form containers, and these containers are classified based on the dosage form ranging from solid to gas formulations. Based on dosage forms, there are solid dosage form containers, liquid dosage form containers, and gas dosage form containers. Solid dosage form containers consist of tamper-resistant packaging such as Bottle seals, strip packages, blister packages, sealed tubes, breakable caps, tape seals, shrink seals and bands, film wrappers, foil paper, child resistant packaging, etc. Liquid dosage form containers consist of glass bottles, ampoules, cartridges, vials, syringes, plastic container, etc.
These containers can either be single-dose or multiple-dose containers, airtight containers, well-closed containers, or light-resistant, depending on the drug packaged. Gas dosage form containers include sprays, vaporizers, prefillable inhalers, aerosols, and nebulizers—the majority of these containers consisting of airtight plastic or glass container with spray pumps. For semi-solids, plastic containers, pressurized packages, and metallic collapsible tubes are used.
Other dosage form containers such as liposome packaging include hermetically sealed borosilicate glass ampoules and narrow and wide-mouth borosilicate glass bottles.
What is the global market size of pharmaceutical packaging?
The size of the global pharmaceutical packaging market plays a valuable role in the global pharmaceutical industry market. The increasing need for drugs has positively affected the growth of the global pharmaceutical market size. Based on Precedence research, the global market size in 2019 was valued at US $98.83 billion.
In the following forecast period from 2020 – 2027, it is expected to experience a compound annual growth rate (CAGR) of 8.6%, surpassing the value of US $191.21 billion by 2027. The growth of the global pharmaceuticals packaging market size has been contributed to by various key factors such as the increasing demand for blister packaging, patient-oriented drug, increasing aged population, rising health awareness, etc. Pharmaceutical packaging and contract packaging companies have significantly propelled the growth of the pharmaceutical industry.
In 2019, the global market was led by North America, with a value share of about 38% due to the presence of leading pharmaceutical packaging companies within that region. Also, the primary pharmaceutical packaging segment had an estimated value share of 75%, and this is expected to continue over the forecast period. The Asia Pacific is expected to witness a rapid growth rate over the forecast of 12% due to the rapid increase in population size.
How many types of pharmaceutical packaging are there?
Each of the categories/levels of packaging has different types, and the nature of these packages are dependent on the drug dosage form – solid, semi-solid, liquid, and gas. There are numerous types of pharmaceutical packaging based on three different categories. A few of them include:
-blister packs
-vial packaging
-ampoules
-prefilled syringes
-latitudes
-plastic bottle
-glass bottle
-cartons and boxes
-plastic pouches
-strip package
-fin sealed package
-shrink seals
Materials used in pharmaceutical packaging
Just as the types of packaging vary, the raw material used in the production of these packaging containments also vary. Various pharmaceutical packaging companies produce different packaging types, and they are made from other raw materials, organic and inorganic. The choice of pharmaceutical packaging material used for the production of packaging containers is usually dependent on the type of drug to be packaged and specific patient needs. In some cases, these materials can be referred to as primary packaging when they directly contact the drug formulation. Some examples of materials used include glass, plastics, elastomeric material such as rubber, etc.
What are the various packaging materials used in the pharmaceutical industries?
Pharmaceutical companies manufacture drug packaging using various raw materials. Due to technology and research advancement, the range of material options continues to increase. As new materials are discovered and chosen for use, factors such as cost, convenience, dosage form, degree of protection required, sterilization methods, drug-material compatibility, and filling methods are considered. Each material considered or chosen for packaging must be a sustainable material to improve safety and drug usage.
Given the different types of dosage forms, stability, and effect of drugs, a wide range of pharmaceutical packaging materials are used, such as glass, rubber, plastics, metals, paper, films, aluminium foil, and laminates, and fibrous material.
What are the main packaging materials used in pharmaceutical industries?
Although there are various packaging materials, there are a few major ones used by almost all pharmaceutical companies and for most packaging. Glass and plastic are some of the most commonly used materials in drug packaging.
Glass has been used for drug packaging for several years now due to the numerous benefits it offers. Glasses (glass bottles) are transparent, economical, have adequate protection power, can be labeled easily, come in various shapes and sizes, and durable. They are thought to possess high protection power due to their inert composition, which eliminates or reduces the chances of drug-material interactions. The most commonly used glass packaging is amber glass because it offers more protection from U.V. rays than clear glass.
Plastics are also commonly used because of their versatility, flexibility, ease of use, durability, and light weightiness. However, the downside to its use is the possibility of drug-material interactions through the transfer of leachable. Some types of plastics used include polypropylene (P.P.), polyethylene terephthalate (PET), high-density polyethylene (HDPE), and several others.
What are the organic packaging materials available that are used in the pharmaceutical industries?
Pharma packaging companies are progressing to the use of eco-friendly, organic, and biodegradable materials for packaging production. This innovation is considered more appealing to consumers, environment-friendly, and a plus for brand reputation.
Organic raw materials used for packaging production can be derived from plants, animals, agricultural feedstocks waste, and marine food processing industry waste. The use of organic and biodegradable materials helps to maintain a safe environment and atmosphere. Some popularly used organic packaging materials include paper, cork, starch, chitin, cellulose, gelatin, collagen, gluten, soy, keratin, etc.
Accountability of pharmaceutical packaging manufacturers
The development, manufacturing, and production process of drugs and drug products are carefully monitored and inspected to ensure that regulatory policies are duly recognized and followed. Pharmaceutical packaging manufacturers are held accountable by regulatory organizations such as the World Health Organization (WHO) and the Food and Drug Administration Agency (FDA).
Accountability of these packaging companies ensures that the raw materials used for the production of packaging and the final products do not affect the stability, safety, and effectiveness of the drug or drug product.
What are the risk factors in the packaging of pharmaceutical products?
Although pharmaceutical packaging is essential and the benefits of pharmaceutical packaging are enormous, there are various risks associated with packaging. With every step of the manufacturing process comes a risk to the drug's safety and integrity. In addition to consumer safety, drug stability, drug delivery, and integrity, other factors such as brand name, reputation, and profitability face various risks associated with packaging.
Risk factors associated with package design and production include wrong labeling, incorrect or incomplete product information, material-product interaction (containment-drug reaction), contamination, poor seal integrity, supply-chain interference, destruction of products as a result of mishandling, incorrect product count or fill-level, etc.
Other factors influence these risks, and they can become sources of paramount concern when these factors come into play. Some of these factors include a non-sterile processing environment, faulty machines and equipment, damaged raw materials, incompetent staff, and many others.
These risk factors put the company's reputation and profits at risk and the health of patients (consumers).
Who regulates the pharmaceutical industry?
Given the importance of pharma packaging and its effect on drugs, drug products, and consumer safety, strict regulatory guidelines and policies are put in place for pharmaceutical manufacturing companies. These regulatory guidelines and procedures are put in place by regulatory agencies and organizations such as the U.S. Food and Drug Administration (FDA) to ensure good manufacturing practice (GMP) in each pharma company. They ensure that pharma companies provide standard packaging systems, through the use of standard pharmaceutical packaging equipment (packaging machine) or medical devices.
Although the regulatory agencies vary by country, they are global pharmacopeia standards set by the World Health Organization (WHO) which are globally acceptable.
The standards and policies created are implemented to ensure material-drug compatibility, prevent contamination, preserve drug integrity, maintain stability, and ensure patient safety. Pharmaceutical packaging standards deal with production location and its sterility, packaging labels, and packaging materials. Some guidelines that have been set in place across regions globally include Anti-tampering regulations issued by FDA, Labelling regulations (Fair Packaging and Labelling Act 1967), and others.
Pharmaceutical and biopharmaceutical supply chain expertise
The pharmaceutical supply chain consists of several government agencies, research organizations, clinics, hospitals, drug manufacturers and distributors, pharmacies, retailers, and regulatory agencies such as the FDA. This chain is responsible for manufacturing drugs, distribution of prescription and OTC drugs, biologics, and generics, and others.
In some cases, insurance companies, HMOs, and GPOs form part of the supply chain or packaging line, which further complicates it.
Due to the complexity of the supply chain, the supply chain's management and regulation may pose a challenge. However, several trusted partners and companies are well-versed and experienced in the management of the pharmaceutical and biopharmaceutical supply chain; a notable example includes Novartis.
Finding the right partners in a supply chain can be effectively carried out when due research and communication are conducted on the key players of the supply chain.
Latest trends in pharmaceutical packaging
Over time old trends have passed and given way to new ones. While the pharmaceutical packaging industry continues to evolve, various trends arise which are shaping and changing the future of the pharmaceutical packaging industry. These latest trends have come to modernize and transform the global pharmaceutical industry for the benefit of the manufacturers and the consumers.
These new trends have actively contributed to the growth of the global pharmaceutical market size in recent years. However, they are also projected to contribute a large percentage of the worldwide market size growth in the nearest future. These key trends have been identified based on collected data and insights gathered by pharmaceutical industry experts.
What are the new trends in pharmaceutical packaging?
The Global pharmaceutical industry has changed rapidly over the years and is still evolving. Due to the increasing population, demand for drugs, and many other factors, the need for new and modernized drugs is on the rise. As a result, there is need for a new pharmaceutical packaging solution and design to accompany the production of these new drugs.
Accompanying new drug developments are recent pharmaceutical packaging trends in the pharmaceutical manufacturing organizations to ensure consumer safety and convenience, manufacturer's profitability, and boost brand reputation.
Some of the newest trends impacting pharmaceutical packaging include Eco-friendly packaging, innovative packaging automation and machinery, sustainability, smart packaging, minute production lots, patient engagement through increased accessibility, and ease of production. Newer innovations directed at meeting patients' needs, ensuring the company's security needs, and maintaining quality include unit dose vials, child-resistant packs, prefilled syringes, and blow fill seal vials (BFS), etc., have also impacted pharmaceutical packaging.
What is new in the world of smart packaging?
Technology is rapidly evolving, and every sector is making efforts to step into the future early, the pharmaceutical industry inclusive. Smart packaging is an outstanding innovation in the pharma industry. With the invention of new pharmaceutical and biopharmaceutical products, pharma companies need to provide new means of storage and transport of drugs and drug products to maintain their integrity.
Wrongly or poorly packaged drugs can pose numerous health risks to the consumer. Also, damage to good packaging leaves drugs and drug products liable to damage and can even pose devastating health risks to the consumers. So, this is where smart packaging comes to play. Smart packaging provides tools that can help preserve pharmaceutical containers and improve consumer cooperation and use. Some new intelligent packaging trends include the use of time-measured indicators, bar code, temperature-oriented indicators, ultra-thin ICS, Near-Field Communication (NFC) tags, and chemicals or biosensors to indicate the safety of the drug.
These packaging innovations drive the brand or pharmaceutical company's success, improve consumers’ health, prevent drug counterfeiting and consumption of these counterfeit or damaged drugs and drug products.
Who are the leading companies operating in the global pharmaceutical packaging market?
Considering the importance of quality packaging and processing, it is no surprise that there is a rising competition between pharmaceutical processing and packaging companies within the pharmaceutical industry. These key players have actively contributed to the growth of the global pharmaceutical packaging market and the pharmaceutical industry.
These companies are considered to be leading players based on their yearly generated revenue, mergers and acquisitions, and their ability to stay at the top of key trends per time. Some of the leading packagers in the global pharmaceutical packaging market include Amcor Limited, Aptar Group Inc., Catalent, Bilcare Inc., ACIC Pharmaceuticals Inc., Marchesini Group S.p.A., COESIA S.p.A., IDEX Corporation, Kȍrber AG, GEA Group, Trucking Technology Limited, Berry Global Inc., Gerresheimer AG, Scott AG, Berry Global Inc., West Pharmaceutical Services, Inc., WestRock Company, and many others.
The importance of the right type of pharmaceutical packaging cannot be overemphasized. This applies not only to pharmaceutical formulations but other products in different industries. It is important to note that a specific packaging material may not be suitable for two different drugs because of its components.
Given the importance of pharmaceutical packaging, it is no surprise that the pharmaceutical packaging and processing company is a significant pharmaceutical industry sector. They largely influence the market growth of pharmaceutical industry.
References
https://blog.drupa.com/de/pharmaceutical-industry-benefit-intelligent-packaging/
https://www.originltd.com/useful-resources/product-information/types-of-pharmaceutical-packaging/
http://eprints.ugd.edu.mk/19277/3/10.%20FORMULATION%20AND%20PACKAGING.pptx
https://www.pharmatutor.org/articles/the-pharmaceutical-packaging-article?amp
https://www.pharmaguideline.com/2018/03/packaging-of-pharmaceutical-products.html?m=1
https://www.idosi.org/mejsr/mejsr12(5)12/16.pdf
References
Upcoming Events
-
CPHI North America 2024
Pennsylvania Convention Center, Philadelphia
07 May 2024 - 09 May 2024 -
-
CPHI South East Asia 2024
Queen Sirikit National Convention Center, Bangkok, Thailand
10 Jul 2024 - 12 Jul 2024
Pharmaceutical Industry Webinars
-
Webinar Exploring Three Technological Trends in the Future of Pharmaceutical Manufacturing
-
23rd May 2024
-
4pm CET / 10am EST
-
-
Webinar Achieving Manufacturing Excellence Through Digital Transformation
-
16th April 2024
-
4pm CET / 10am EST
-
-
Webinar Made in Africa: What’s Driving Pharma Manufacturing
-
28th March 2024
-
4pm CET / 10am EST
-
-
Webinar Case Study: Risk Management for Annex 1 Sterile Production EMS
-
28th February 2024
-
4pm CET / 10am EST
-
-
Webinar Innovative Strategies for B2B Pharma Marketeers: Driving Value through Content
-
20th February 2024
-
4pm CET / 10am EST
-
-
Webinar Revolutionizing Pharma: Data and AI Unleashed
-
18th January 2024
-
4pm CET / 10am EST
-
-
Webinar Optimal Temperature: Elevating Biologics Cold Chain Excellence
-
16th January 2024
-
4pm CET / 10am EST
-
-
Webinar Market Outlook – The Biggest Pharma Trends of 2024
-
12th December 2023
-
4pm CET / 10am EST
-
-
Webinar The Next Frontier – Emerging Opportunities in the LATAM Pharma Market
-
21st November 2023
-
4pm CET / 10am EST
-
-
Webinar Vistamaxx™ MED - imagine the possibilities for healthcare product performance
-
10th October, 2023
-
4pm CET / 10am EST
-
-
Webinar Co-processing: A Multifaceted Approach for Enhancing Density & Powder Flow
-
19th September 2023
-
4pm CET / 10am EST
-
-
Webinar The Outlook for Cell & Gene Therapy Manufacturing
-
28th June 2023
-
4pm CET / 10am EST
-
-
Webinar Contract Packaging Outlook: Growth Trends within the Commercial Packaging Sector
-
25th May 2023
-
4pm CET / 10am EST
-
-
Webinar The HPAPI Boom: How Pharma is Rising to the Manufacturing Challenge
-
23rd February 2023
-
4pm CET / 10am EST
-
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance































-comp241444.jpg)



















































































































.png)
.png)
.png)
.png)




.png)









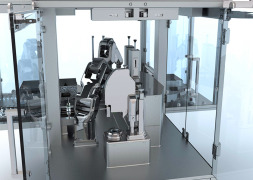






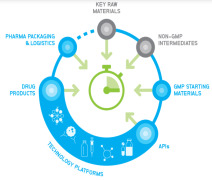








.png)
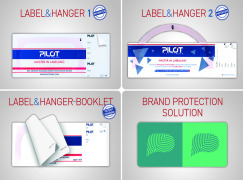








































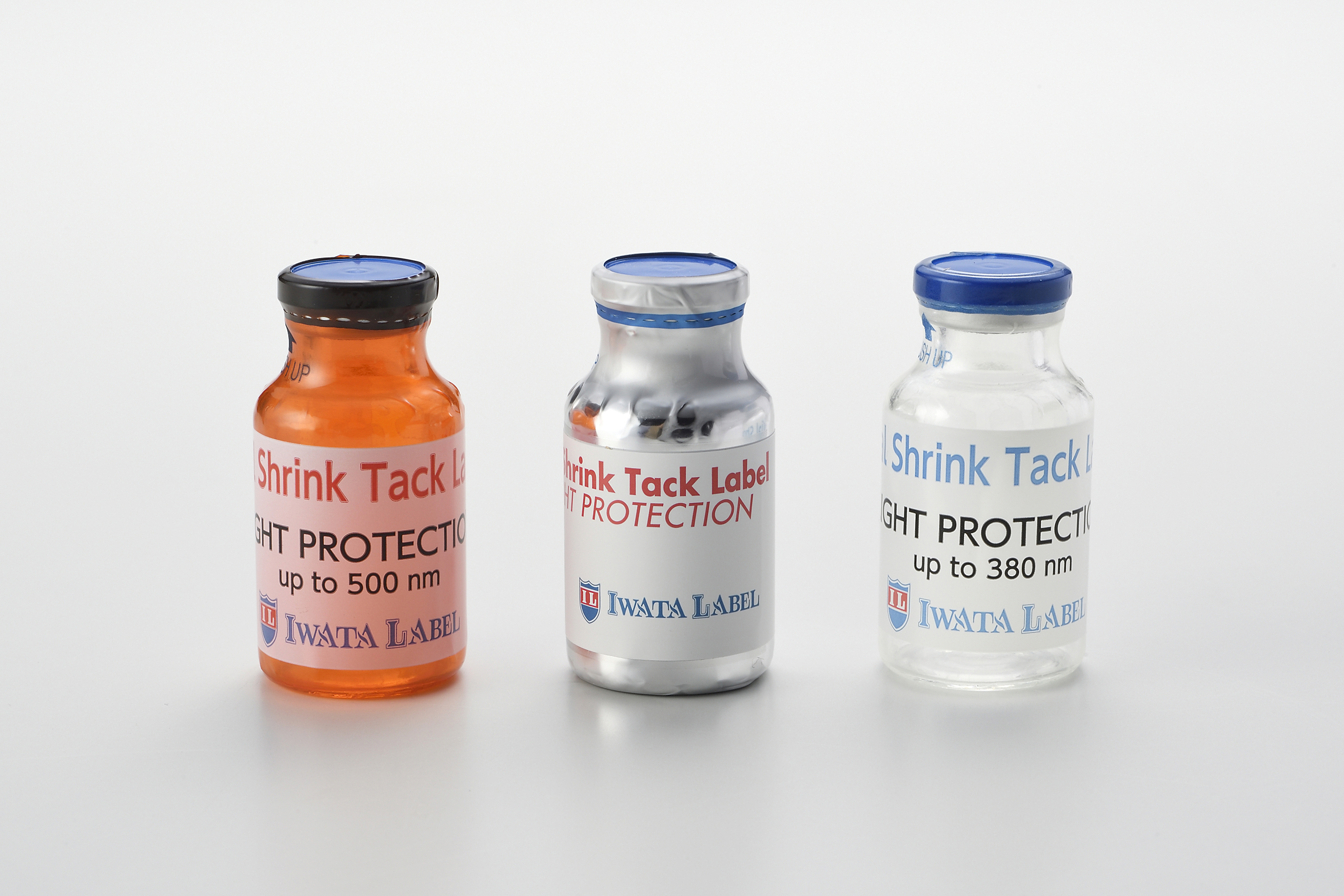











.png)













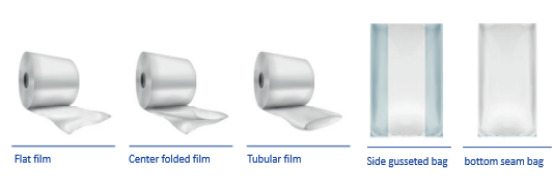

















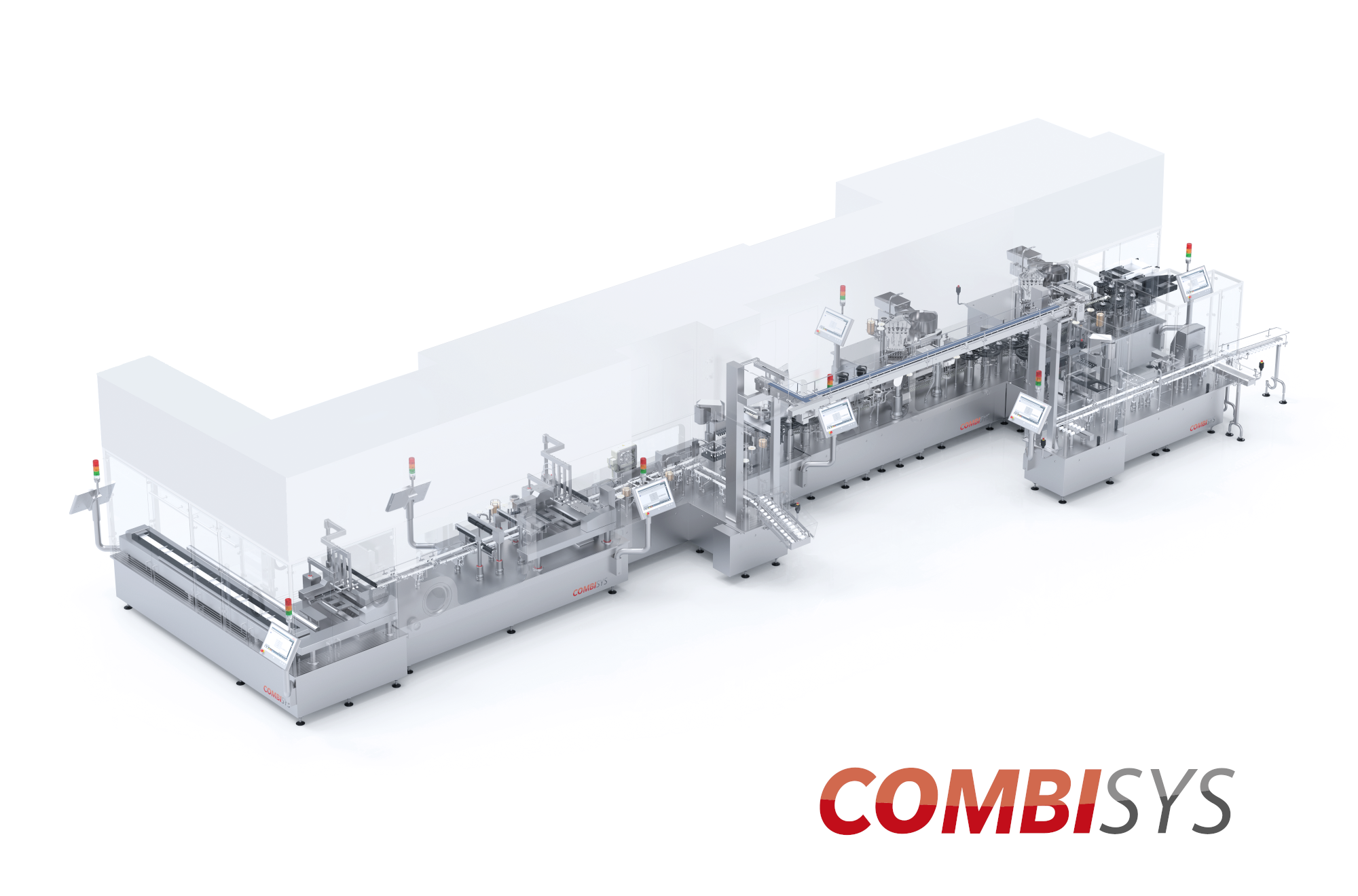



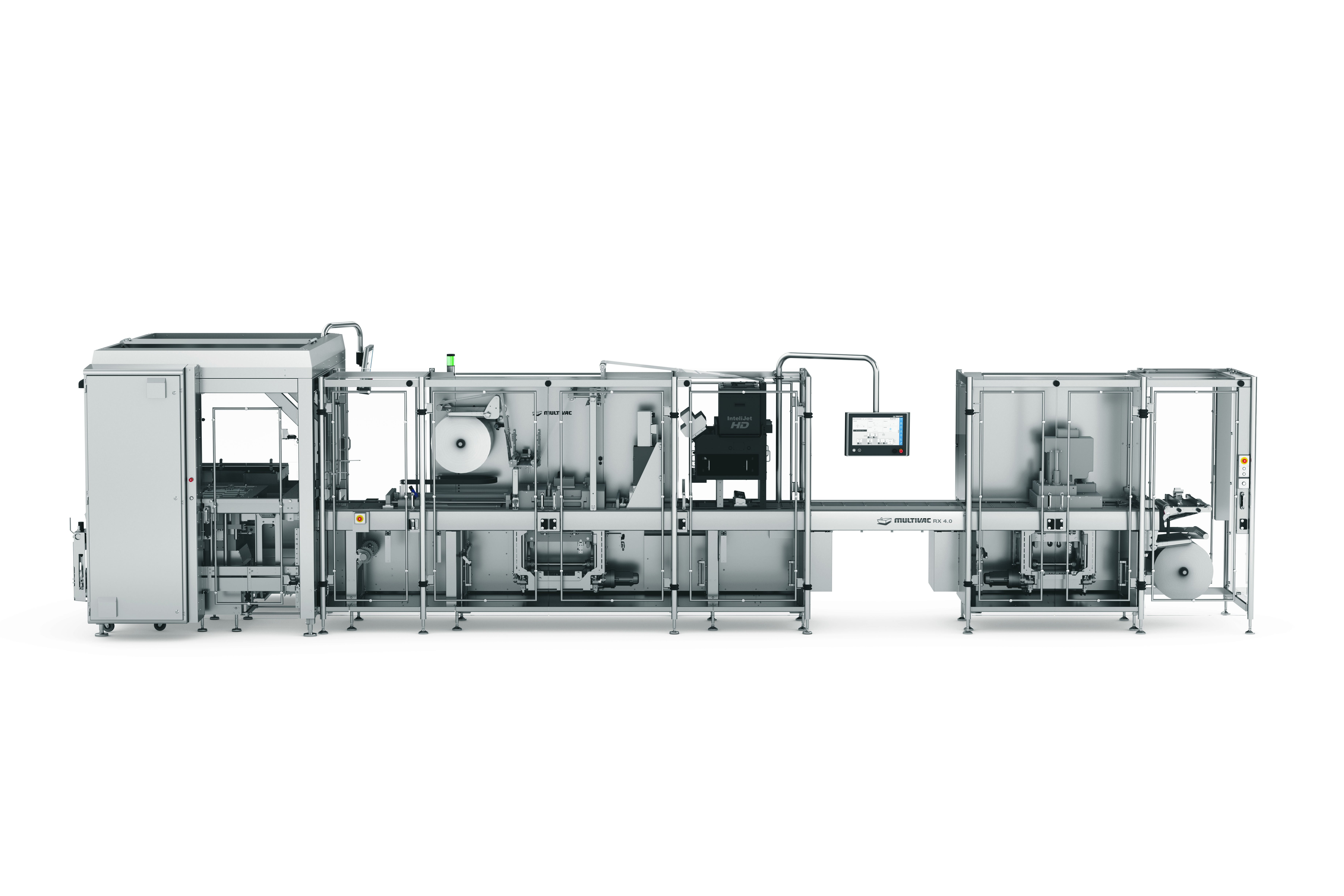














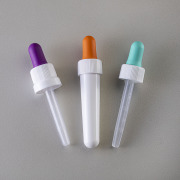












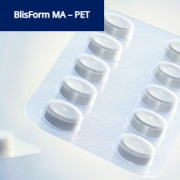
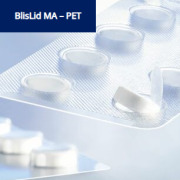




















.png)


.png)



.jpg)