Secondary Pharmaceutical Packaging
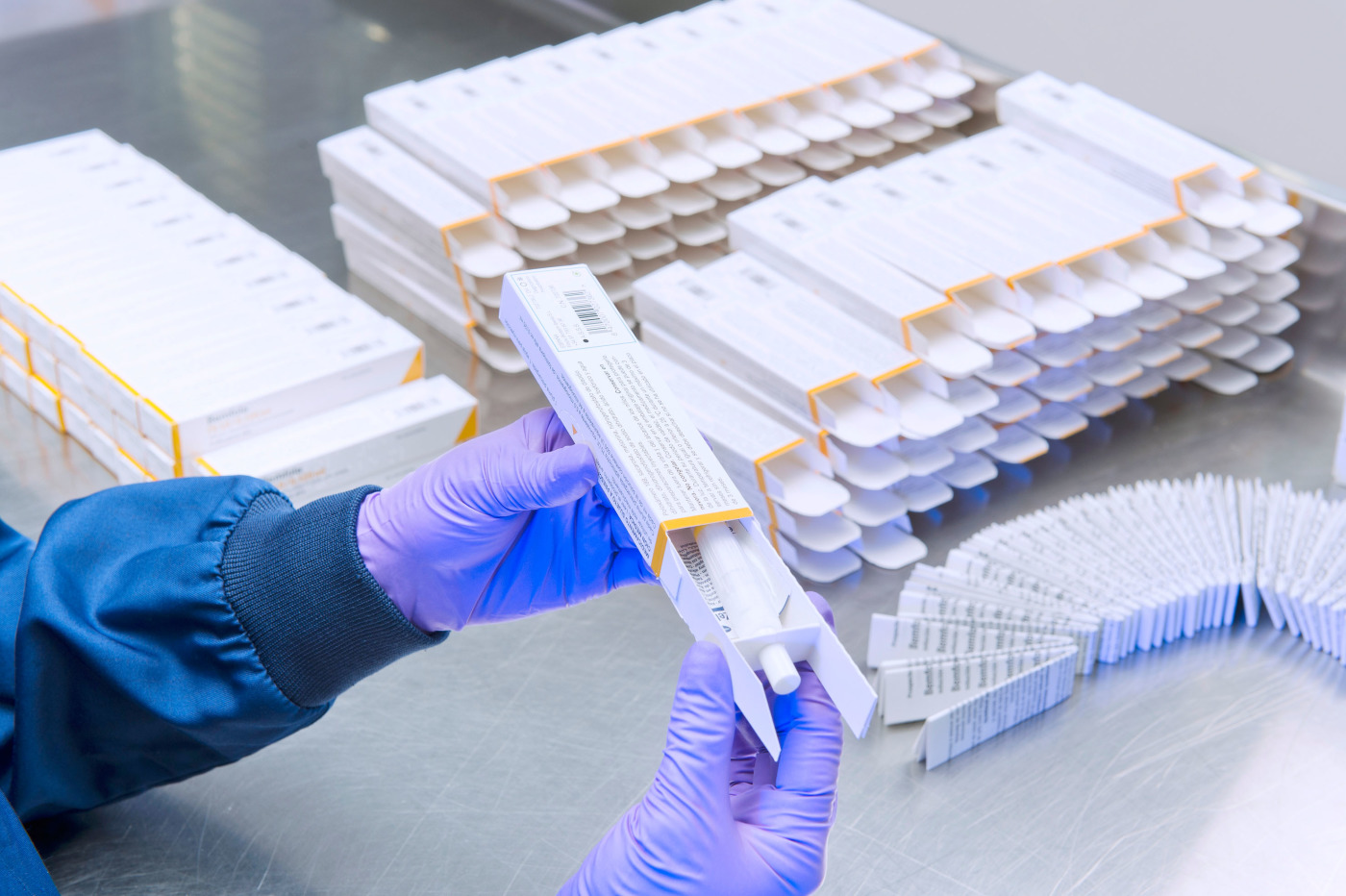
Product Description
allpack group ag

-
CH
-
2019On CPHI since
-
3Certificates
-
100 - 249Employees
Company types
Categories
Specifications
allpack group ag

-
CH
-
2019On CPHI since
-
3Certificates
-
100 - 249Employees
Company types
More Products from allpack group ag (2)
-
Product Primary Pharmaceutical Packaging
Primary Pharmaceutical Packaging
Blister lines for solid dosage forms
Tablets, sugar-coated tablets, capsules, oblongs, syringes, etc.
Films/foils: PVC, PVC/ PVdC, PVC/PE/PVdC, PVC/ACLAR/PVC, COC-Laminates, PP, PET, Tyvec or alu/alu (with/without childproof seals)
... -
Product Serialization and Aggregation
On the basis of GMP-compliant processes – certified by the FDA and Swissmedic and in accordance with PMDA standards (Japan) – our packaging operations satisfy the highest standards of quality.
The packaging process - implemented by allpack group ag - with continuous serialization and...
allpack group ag resources (2)
-
News Russian Crypto Code
We have extended our #pharmaceutical #packaging competence to include cryptographic coding for the Russian market.
Our lines as well as printing and camera control systems are equipped with the necessary add-ons, connection to external IT-systems has been successful.
This job is done. New serialization and aggregation challenges based on individual country requirements are already in our pipeline. #healthcare #pharmaindustry #pharmaceuticalman... -
Video Corporate Image Video "allpack group ag"
allpack – a leading service provider for pharmaceutical packaging
We are a GMP-compliant pharmaceutical contract packaging organisation (CPO) that has the passion and drive to create and produce well suitable and cost-efficient packaging solutions for our customers. Join us on a journey of discovery in our world of pharmaceutical contract packaging. All you need is allpack.
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance







