Pharmapack 2023: Session interview – BD Pharmaceutical Systems on de-risking combination product development

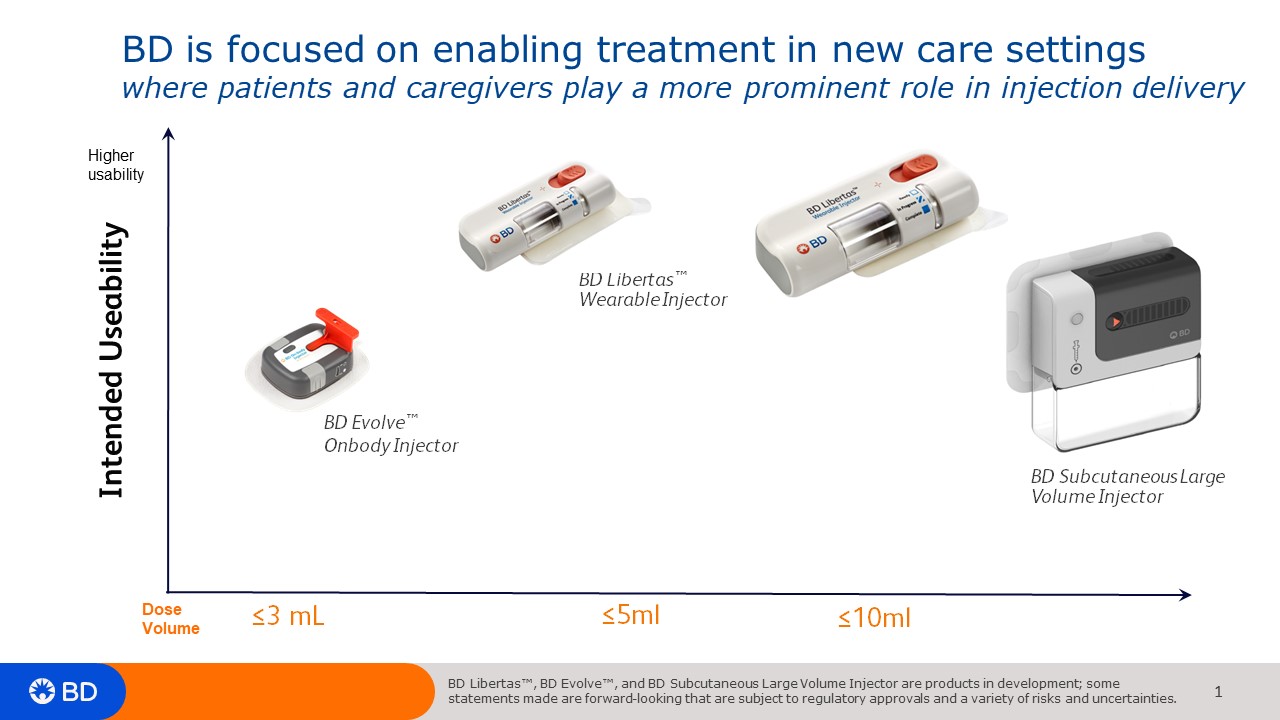
The BD Libertas 5ml & 10ml wearable
Pharmapack 2023 showcased a number of sessions on the key topics in the industry, from conference sessions to workshops, presented by experts in the field.
After attending the session 'De-risking combination product development for large volume biologics: a pharma partner’s perspective' from BD Medical, Digital Editor Lucy Chard caught up with Adam Kalbermatten to find out more.
Please could you introduce yourself and your role at BD Medical?
My name is Adam Kalbermatten. I lead our advanced drug delivery commercial development team at BD Medical – Pharmaceutical Systems (NJ, USA). My team is responsible for working with pharmaceutical companies globally on their advanced drug delivery needs. This could be on anything from hand held safety devices, pen injectors, autoinjectors or more advanced wearable products. Ideally, we’re working with pharma companies to get their drug-device combination products to market and providing different services along the way to help that process go as quickly and seamlessly as possible.
Could you please give a quick overview of your presentation?
We talked about de-risking large volume subcutaneous delivery and what it looks like from a pharmaceutical partners’ approach and the different elements that are considered in developing the combination product. If a drug will be put it into a wearable large volume injector, there are things that need to be considered early on in the development process. It’s an integrated process that needs to be started early, in the Phase I/II process. These days it is very challenging to fit a complex drug into 1 mL or 2.25 mL autoinjector when you have to navigate viscosity, concentration of the drug, and volume of the drug that’s delivered. A wearable injector allows you flexibility with those trade-offs, where you could increase the volume delivered and make it less viscous, for example, which could make it less painful for the patient. You could also make it a longer delivery so rather than the patient having to hold an autoinjector for 10 seconds, they could wear an injector for 10 minutes, hours or even days.
You discuss working to understand the patient journey throughout the injection process, why is this information so vital as a pharmaceutical manufacturer?
We recognise that for patients to stay adherent to our customer’s therapy, our product must be useable – first and foremost. Involving that patient and keeping them at the forefront of the design considerations being made is important – and helps us find the right balance between useability and the manufacturing process. As an example, we could look at a device that the patient must fill, which puts the burden on them to prepare, and then inject, or could we make it a prefilled, preassembled device where all they have to do is peel off an adhesive, stick it to their body, and then start the injection. A prefilled, preassembled device could be preferred if the sequence of preparation events could take upwards of 20–30 minutes to prepare – that is a significant amount of time out of someone’s day. There’s a lot of safety considerations for that too, many patients are in their homes, administering these medicines at their kitchen table, these places are naturally not sterile. So it may be better to have everything ready to administer, and you don’t have to worry about contamination.
What are some of the biggest risks that large volume biologics manufacturers face?
The biggest risk is timeline delays in getting to market. These manufacturers are under immense pressure to develop drugs, get them to market, get them to patients, and they have financial considerations as they are doing this as they need to be sustainable entities and generate some sort of revenue to feed these development programmes. So they may leave patient centricity to the last step, and that inevitably can cause delays. They need to get to that earlier so that they’re not having to make trade-offs in timeline compared to doing the right thing for the patient.
How do you use partnerships, and how important are they, in working together to mitigate these risks?
Drug delivery can be complex – particularly in rapidly growing areas like biologics. To best meet our customers’ needs, we certainly do prioritise collaboration. And we’re thinking about that in different ways now. For example are there CDMOs, CROs that we should be working with. When we are trying to develop new products with pharma, there is often a consideration of how are they going to fill/finish these? How are they going to assemble them? If a CDMO network doesn’t exist or if a pharma company isn’t going to do it internally, that needs to be built out in advance. BD Medical – Pharmaceutical Systems has a lot of that tooling in place and we can do that at a very small scale, in an R&D-based environment. When it comes to introducing it into an aseptic environment, we aim to have everything ready to go so our CDMOs can partner with us even before the clients are ready to do projects, that way the market is proactively ready to support these new technologies in the wearable space.
It helps in scaling up too, and there’s other things that BD has realised, where there might not be something that we have in our wheelhouse but that we could acquire inorganically into our portfolio. Services are a good example of that. Recently we acquired a company called ZebraSci, which does all combination product development testing for drug device combo products, everything from container/device characterisation studies all the way through design verification and full functional stability in a GMP environment – we make that available for our clients. We are not just developing a delivery system together, but we’re providing drug device combination product development services to help make the development and commercialization process go faster – pharma doesn’t have to do it all themselves.
What are the main drug delivery technologies that help to overcome these issues that we need to look out for?
Given the complexity of molecules, it’s very important to ensure that we’re utilising the latest container technology. For example, using diving nozzle applied silicone so that we have a very even distribution on our containers. Then as BD Medical – Pharmaceutical Systems is a primary container company, we’re also evaluating how best a device could work with that container, making sure we don’t have any container/device interaction issues. This may be overlooked and can often lead to development or commercialisation delays. We’re designing a system to work from the start – so from a device and container side those are really two of the main technologies.
Do you have to make considerations, as a lot of companies are at the minute, for sustainability?
We view sustainability as a portfolio of complementary initiatives and actions that help us achieve our purpose of advancing the world of health™. We see the modern social and environmental challenges our world faces as opportunities to make a difference while strengthening our company. This often means finding the right balance between patient’s useability of our devices and overall product sustainability.
Currently, we are working with Ecotron. Ecotron is a Horizon Europe project aiming to improve the sustainability, circularity, and recyclability of printed electronics (PE). Our BD Libertas product has been nominated and accepted as a project to look at a wearable injector and how we can make the electronics and circuitry integrated into the device compostable and easily disposable so that it can be broken down in a very environmentally friendly fashion.
Any further comments?
We are committed to drug delivery excellence and partnering with pharma and biotech companies – large and small – throughout the combination product development process to help enable customer success and help accelerate time to market!
Related News
-
News On Track at Pharmapack 2024 - The Track Sponsor interview: BD Pharmaceuticals
January 2024 brings both a new year and Europe’s leading packaging and drug delivery event. Bringing the world’s experts in pharmaceutical packaging together in Paris, France, Pharmapack 2024 brings exciting opportunities to learn and colla... -
News CPHI Online Webinar Series – The CPO Perspective: Driving Healthcare Sustainability
Pharmaceutical contract packaging organisations (CPOs) offer organisations broader expertise in pharma packaging, specialist skills, and flexibility with time, efficiency, and costings for pharmaceutical companies lacking in-house packaging expert... -
News Pharmapack Europe 2023 Award Winners – ATB West for Patient Centric Design
We interview the winners of the Pharmapack Europe Awards 2023, who were chosen by a jury, and each developed an innovative solution in the categories of Drug Delivery Innovation, Packaging Innovation, Sustainability Initiative, Eco-Design, and Patient-... -
News Pharmapack 2023: Session interview – Terumo on integrated CDMO solutions
Pharmapack 2023 showcased a number of sessions on the key topics in the industry, from conference sessions to workshops, presented by experts in the field. -
News Pharmapack 2023 – Take the Start-Up innovation tour
At Pharmapack 2023 in Paris we debuted a new and improved Start-Up Hub. The Hub included several start-up companies we are looking to support and make the event more accessible. -
News Pharmapack Europe 2023 Award Winners – PACKSYS GmbH for Packaging Innovation
We interview the winners of the Pharmapack Europe Awards 2023, who were chosen by a jury, and each developed an innovative solution in the categories of Drug Delivery Innovation, Packaging Innovation, Sustainability Initiative, Eco-Design, and Patient-... -
News Women in Pharma – Karin von Kienlin Pharmapack 2023
This year at Pharmapack 2023 in Paris, France, the CPHI Online team interviewed several leading women in the pharmaceutical industry about what it means to be a woman working in this field. -
News Women in Pharma – Asmita Khanolkar Pharmapack 2023
At Pharmapack 2023, the CPHI team interviewed several leading women in the pharmaceutical industry about what it means to be a woman working in this field. Ahead of International Women’s Day on March 8, 2023, we are proud to provide a p...
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance

.png)