Antibodies
Antibodies Companies (45)
Antibodies News
-
News Pfizer forges ahead with blood cancer therapy after approval from FDA
Pfizer gains accelerated approval from the US FDA for their new bispecific antibody therapy for multiple myeloma, set to address an unmet need for patients. -
News Alzheimer's drug Leqembi receives go ahead from the FDA to be used in patients
Eisai achieves FDA approval for it's Alzheimer's disease drug Leqembi, after rigorous testing in clinical trials show it reduces the size of characteristic plaques in the brains of patients. -
News Lassa virus antibody cocktail could be the first step in developing a vaccine
A research team from La Jolla Institute for Immunology in California, USA, have identifed a trio of antibodies that could be used to neutralize the Lassa virus -
News New dual-action vaccine against original SARS-CoV-2 strain and Omicron variant
Moderna’s novel bivalent vaccine is the first of its kind to be approved by the UK Medicines and Healthcare Products Regulatory Agency in the fight against the COVID-19 pandemic and SARS-CoV-2 variants.
Antibodies Products (41)
-
Product Biologic Quality Control and Release Testing
We deliver responsive QC analysis for complex biologic products from our cGMP laboratories. Our scientists develop and validate methods or perform technology transfer of a sponsor's method for a wide range of analytical methods required for batch release testing. We also routinely carry out testing to Phar...
-
Product Biosimilars Portfolio
• Pegfilgrastim (Filed with EMA) • Filgrastim (Filed with EMA) • Trastuzumab (Filed with EMA, MHRA, Received recommendation for marketing authorization in India) • Bevacizumab (Filed with MHRA) • Omalizumab (Global PhIII ongoing) • Denosumab (Global PhIII ongoing) • Ranibizumab (Global PhIII ongoing...
-
Product Lead Discovery and design
Abzena’s antibody discovery team collaborates with you to build the foundations of a successful program and de-risk your development path. We deliver expert assessments of target molecules, immunization strategies and antibody screening cascades that inform your selection of the best candidates for develo...
-
Product A CDMO PARTNER FOR LIFE
FUJIFILM Diosynth Biotechnologies is an industry-leading cGMP Contract Development and Manufacturing Organization (CDMO) supporting the biopharmaceutical industry in the development and production of biologics, vaccines and cell and gene therapies. Our focus is to combine technical leadership in process d...
-
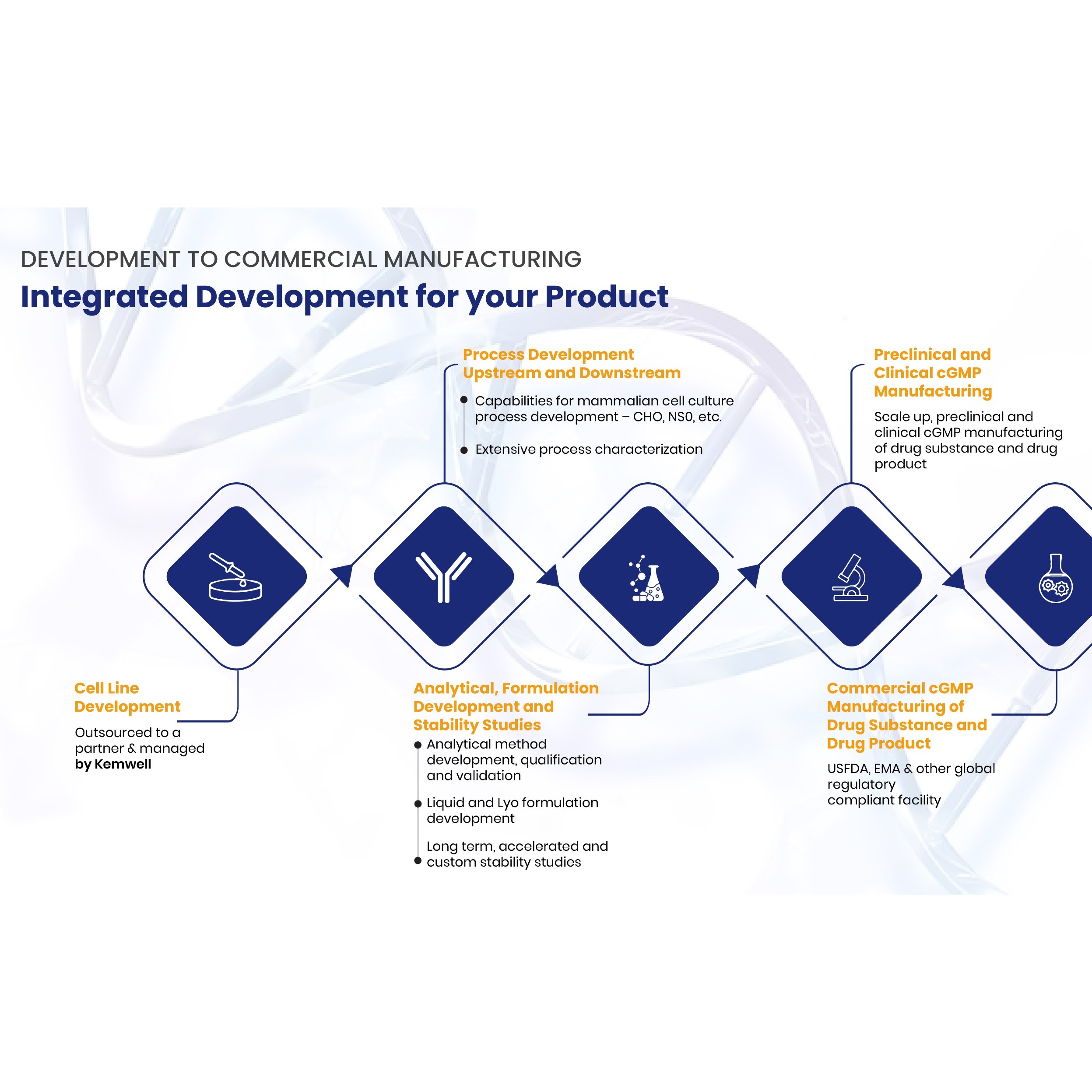
Product Kemwell Biopharma
SERVICES Kemwell provides integrated development and manufacturing services for companies that require one-stop solution for mammalian cell-culture based protein therapeutics The team is experienced to undertake end-to-end activities right from cell line development till cGMP clinical and commercial manufa...
-
Product 1 GHz NMR
New Evo 1 GHz NMR fits in 1 story building, uses 70% less helium. Ideally suited to characterise complex molecules such as RNA, oligonucleotides, peptides, antibodies and more!
-
Product cGMP Protein and Antibody Purification
KBI Biopharma has extensive experience developing purification processes for a wide variety of biotherapeutics, including: -Monoclonal antibodies-Bispecific antibodies -Enzymes -Fc fusion proteins -PEGylated proteins -Complex glycoproteins KBI supports 40+ annual programs ranging from FIH (First-in-Huma...
-
Product Antibody Drug Conjugates (ADC)
hen selecting AbbVie Contract Manufacturing, you are partnering with a leading developer and manufacturer focused on accelerating and mitigating risks to program timelines and on efficiently fast-tracking your program to completion. AbbVie’s mAb and ADC state-of-the-art facility and expert scientific team ...
-
Product API-Epalrestat
Product name: Epalrestat
CAS NO: 82159-09-9
Appearance: Yellow to orange red crystalline powder
Specification: JP
Documentation: DMF, GMP
Application: Diabetes
Package: 25kg/drum

-
Product Filgrastim (g-csf)
Axxo gmbh offers biopharmaceutical products which includes filgrastim (g-csf). It belongs to recombinant human proteins products category. It is for post-cancer treatment. Contact us for more information.
-
Product Doxylag 100mg (Doxycycline) Caps
Doxycycline, the active ingredient of Doxylag, is an antibiotic that exhibits a wide spectrum of antimicrobial activity against both Gram-positive & Gram-negative bacteria.
-
Product Biologic drug substance CDMO services
From pre-clinical development to commercial supply, Patheon by Thermo Fisher Scientific is an industry leader in the development and manufacture of mammalian cell culture drug substances. Patheon offers biotech and pharmaceutical companies the ability to pursue opportunities around the globe with a fully i...
-
Product Walk in Cooling Cabinet.
The Walk-In Cooling Cabinets / cold rooms are designed according to ICH guidelines, WHO, MCA and USFDA requirements to maintain uniform conditions. They are superior in airflow distribution, temperature control technology, cabinet construction and are manufactured as per cGMP regulations.
Biggest Walk...
-
Product Oxacillin sodium compacted
Centrient Pharmaceuticals provides wide range of semi-synthetic penicillins which includes oxacillin sodium compacted. Characteristics: granules obtained by means of dry compaction of the crystalline powder without adding any excipients. Application: the product is suitable for the manufacture of capsules ...
-
Product Antibody Drug Conjugates
Syngene’s Antibody Drug Conjugate (ADC) unit brings together an array of multidisciplinary capabilities in antibody generation, linker and payload chemistry, conjugation chemistry, in vitro and in vivo oncology in an integrated platform.
Upcoming Events
-
CPHI Middle East 2024
Riyadh Front Exhibition & Convention Center Riyadh, Saudi Arabia
10 Dec 2024 - 12 Dec 2024 -
Pharmapack Europe 2025
Paris Expo, Porte de Versailles - Hall 7.2 | Paris, France
22 Jan 2025 - 23 Jan 2025 -
CPHI Japan 2025
East Halls 4, 5 & 6, Tokyo Big Sight, Tokyo, Japan
09 Apr 2025 – 11 Apr 2025
Pharmaceutical Industry Webinars
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance

















-comp282768.jpg)






















.jpg)
.jpg)

.jpg)
.jpg)










































.png)







.png)

.png)



.jpg)


