Aralez Bio
About Aralez Bio
Certifications
Categories

-
US
-
2021On CPHI since
-
1Certificates
-
1 - 24Employees
Company types
Primary activities
Meet us at
CPHI North America 2024
Pennsylvania Convention Center, Philadelphia
07 May 2024 - 09 May 2024
Products from Aralez Bio (5)
-
Product Other Aromatic Analogs and Derivatives
Our enzyme platform creates combinatorial diversity by allowing other aromatic subtrates to act nucleophiles and form other aromatic amino acid analogs. -
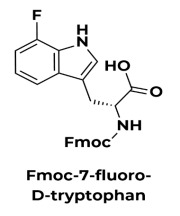
Product D-amino acids
As peptide therapeutics experience explosive growth, the need to address the pharmacokinetic liabilities of canonical L-amino acids grows. D-amino acids are less susceptible to enzymatic degradation and, when placed appropriately in a peptide or protein sequence, can confer additional selectivity and potency. -
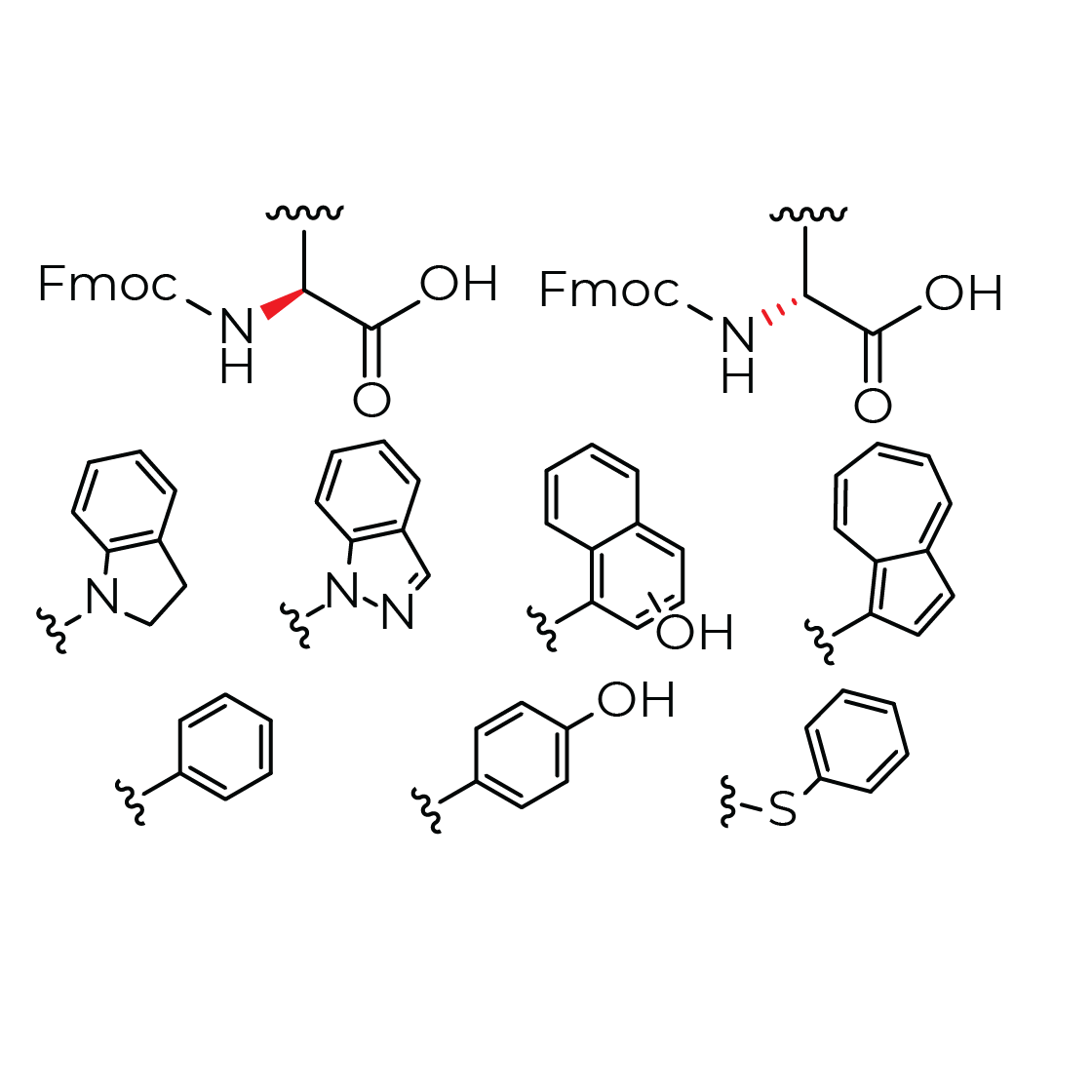
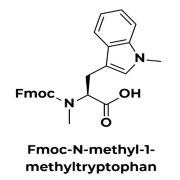
Product N-methylated amino acid analogs
N-methylation (and N-alkylation more broadly) increases steric hindrance around the peptide bond, reducing the peptide’s entropy and increasing affinity and selectivity. This modification also confers protease resistance, and the disruption of hydrogen bonds can improve bioavailability.
Access to N-me... -
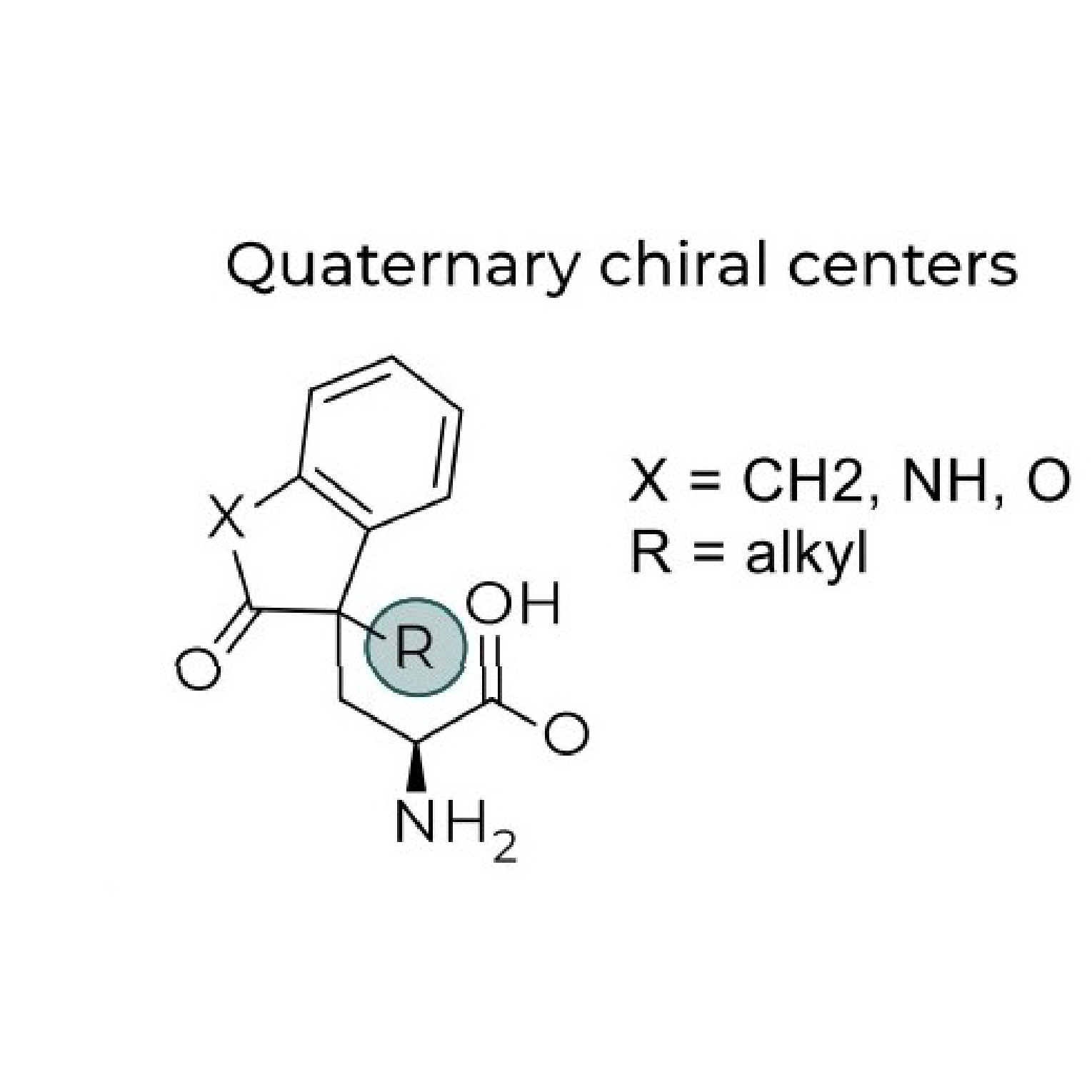
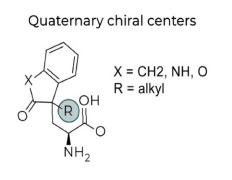
Product Quaternary Chiral Center Amino Acids
Previously hard to access, these compounds with all carbon chiral centers are more stable than tertiary chiral centers. Without using enzymes, these structures are very difficult to make using traditional/conventional chemistry.
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance