Sera and Vaccines
Sera and Vaccines Companies (31)
Sera and Vaccines News
-
News BioNTech to begin mRNA vaccine manufacturing in Rwanda by 2025
German biotechnology company BioNTech has stated their intentions to begin production at their mRNA vaccine factory in Rwanda by 2025, which will mark the first foreign mRNA vaccine manufacturing site on the continent of Africa. -
News Revolutionising cancer treatment with mRNA-based therapeutics
Global market for mRNA-based oncology therapeutics expected to reach USD $2 billion by 2029, with promising results for the combination of mRNA candidates with immune checkpoint inhibitors to treat solid tumours. -
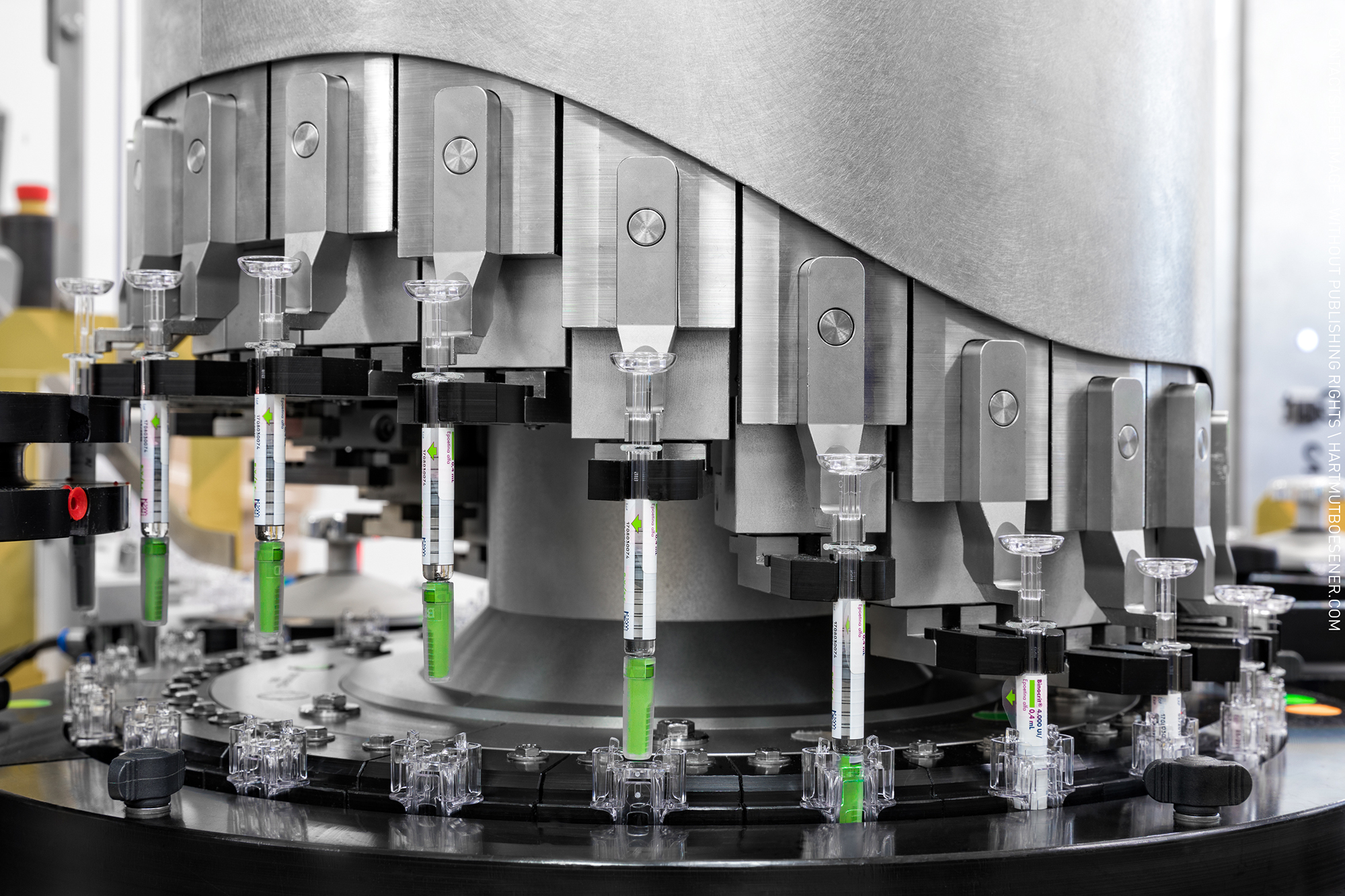
News Delivering on mRNA-based therapeutics: innovations in applications and packaging
Since the onset of the COVID-19 pandemic, the innovative potential of mRNA vaccines and therapeutics has raised questions regarding their manufacturing, packaging, and storage/transportation. Learn about how the pharmaceutical supply chain is meet... -
News Novavax will provide adjuvant Matrix-M for vaccine research with 3-year partnership
Novavax, developers behind the Matrix-M adjuvant, have entered into an agreement with the Bill and Melinda Gates Medical Research Institute to provide the adjuvant in preclinical vaccine research.
Sera and Vaccines Products (118)
-
Product Hepatitis A Vaccine (Human Diploid Cell), Inactivated - Healive®
• Hepatitis A Vaccine (Human Diploid Cell), Inactivated – Healive® Launched in 2002
Hepatitis A & Hepatitis B Combined Vaccine – Bilive®
Launched in 2005
Healive® is:
Prequalified by the World Health Organization.
Provides susta...
-
Product Packaging, Quality Control, Storage, Cold Chain
Within the contract development and manufacturing, IDT Biologika GmbH provides a wide range of other pharmaceutical services for our clients products, which include- Labeling & Packaging of vials, prefilled syringes, autoinjectors - Serializiation (Track & Trace) - Quality Systems - Cold Chain up to - 80- ...
-
Product Media for Vaccine Production
WakoVAC is FUJIFILM Wako Pure Chemical's Serum Free Cell Culture Media Series for Vaccine Production.The lineup includes for MDCK cells, Vero cells and Insect cells.A series of media with very high performance in cell growth. We are especially aiming to support vaccine manufacturing with our quality prod...
-
Product Trastuzumab Injection
[Dosage form]: Liquid for injection [Specification]: 150mg/vial; 440mg/vial (pipeline)
[Indication]: Indicated for Metastatic breast cancer, Early breast cancer, Metastatic gastric cancer.
[Administration]: Intravenous injection
[Shelf life]: 24 months
-
Product Pharma Latch Hollow
The Pharma Latch Hollow is a sterile-packaged, single-use, disposable medical device intended for use with any liquid formulation approved per the intradermal route of administration.
The Pharma Latch Hollow is compatible with standard luer-lock and slip syringes and can inject high volume and...
-
Product INACTIVATE - I22
INACTIVATE / I22 is a device for the inactivation of pathogens & cells of any kind. By low energy electron irradiation (LEEI) the genetic material of the pathogens & cells is reliably destroyed. The I22 device is suitable for research purposes and small industrial batches. Example use-cases are: Vaccine ...
-
Product Adjuvant Systems for Vaccines
We enable next-generation vaccine development through a diverse portfolio of world-leading vaccine adjuvants, immunomodulators and adjuvant formulations.
We passionately develop new adjuvant technologies and partner to develop the vaccines of tomorrow.
Our extensive expertise in immunology and fo...
-
Product API-Dextran 20
Product name: Dextran 20
Normative Mw: 20000
CAS NO: 9004-54-0
Appearance: White Crystal powder
Specification: BP, EP, USP, CP
Documentation: GMP, DMF, EU written confirmation
Application: Blood Volume Expander, Organ...
-
Product Biosafety Testing
Life-saving medicines are heavily regulated during development, manufacture and distribution. To fulfill regulatory requirements, the biopharmaceutical industry is increasingly looking for independent service providers who can deliver comprehensive characterization solutions on one site.
SGS’s glo...
-
Product Process Equipment & Systems
Terlet is an experienced specialist in developing and manufacturing high quality food processing machinery and food processing systems. Terlet provides solutions for all sorts of (liquid) food processing, such as Stirring, Mixing, Heating, Cooling, Vacuum gassing, Filling (e.g. bag-in-box) & ...
-
Product ABHAYRAB
Abhayrab is a purified Inactivated Rabies Vaccine prepared on Vero cells and is used for the prophylactic or post exposure immunization against Rabies. The vaccine is prepared using L.Pasteur 2061/Vero Rabies strain and is exported widely to 40 countries worldwide. Each pack consists of 1 dose vial along w...
-
Product Combipack Of Snake Venom Antiserum
Premium Serums & Vaccines Pvt Ltd offers a wide range of products which includes combipack of snake venom antiserum. Content: it has indian cobra venom (naja naja), common krait venom (bungarus caeruleus), russell’s viper venom ( vipera russelii ), saw scaled viper venom (echis carinatus), cresol (preserva...
-
Product Japanese encephalitis vaccine for human use
Liaoning cheng da biotechnology co ltd offers a vaccine technology which includes japanese encephalitis vaccine for human use. It is a freeze-dried or liquid preparation and produced from the japanese encephalitis strain-p3 strain based on continuous vero cells culture. It is used for the prevention of ...
-
Product Walk in Cooling Cabinet.
The Walk-In Cooling Cabinets / cold rooms are designed according to ICH guidelines, WHO, MCA and USFDA requirements to maintain uniform conditions. They are superior in airflow distribution, temperature control technology, cabinet construction and are manufactured as per cGMP regulations.
Biggest Walk...
-
Product Walk-In Deep Freezer (up to -30°C)
The Pharmaceutical & allied industries use Deep freezers for effective storage of drug products, life-saving vaccines and samples for diagnosis, but are also perfectly suited for temperature tests and aging subjected at sub-zero temperatures.
They can be used for storage of fresh and...
-
Product PhytoChol®: Plant-derived cholesterol
Plant-derived cholesterol for mRNA delivery, gene therapy and cell culture: Our plant-derived PhytoChol® provides you with a secure and stable supply of synthetic cholesterol. Evonik supplies PhytoChol® in two grades that are tailored to the specific needs of injectable drug delivery and biopharm...
-
Product Sodium Succinate anhydrous
Dr. Paul Lohmann® offers a wide range of high-quality Mineral Salts suitable for the final vaccine formulation. Since Mineral Salts stabilize biologics and vaccines during processing, storage and application, they are an essential part of the entire biomanufacturing process. Please contact us f... -
Product Vaccine adjuvants
Imcd india pvt. Ltd. provides a wide range of products which includes vaccine adjuvants. It can stimulate the innate immune system, significantly improve the effectiveness of antigen-based vaccines and has no antigenic effect itself. Contact us for more information.
-
Product Human Albumin
The main component of this preparation is human albumin, which is prepared by cold ethanol fractionation of human plasma from healthy donors. The manufacturing process of human albumin contains a step of heating the final solution at 60℃ for 10 hours to inactivate the viruses present in it. It contains...
-
Product Vaccine & Biotech Fill-And Finish
Pharma Access offers wide range of products which includes vaccine & biotech fill-and finish. It is perilous in biotech product manufacturing where the product value, and hence the risk, is high. Because of its criticality, fill finish has historically been an area with a focus on “proven solutions” and le...
Upcoming Events
-
CPHI Middle East 2024
Riyadh Front Exhibition & Convention Center Riyadh, Saudi Arabia
10 Dec 2024 - 12 Dec 2024 -
Pharmapack Europe 2025
Paris Expo, Porte de Versailles - Hall 7.2 | Paris, France
22 Jan 2025 - 23 Jan 2025 -
CPHI Japan 2025
East Halls 4, 5 & 6, Tokyo Big Sight, Tokyo, Japan
09 Apr 2025 – 11 Apr 2025
Pharmaceutical Industry Webinars
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance


























.png)
.png)

















































.png)







.png)

.png)



.jpg)


