Beyond the platform – applying modularity to autoinjector development

SHL’s Molly® and Molly® 2.25 mL autoinjectors are built upon a proven modular platform technology to support shorter development timelines and increased flexibility in device design and production.
Injectable drug delivery systems have become an important and integral part of modern medicine, owing to various key drivers such as human health and well-being, drug development and delivery, as well as patient safety and usability. Injectable therapy has at the same time been met with an increased focus on self-administration, mainly due to the added benefit of patient convenience, but also due to the growing pipeline of biological and biosimilars drugs.
For the past decade, preconfigured or platform self-injection devices such as autoinjectors have been heavily sought after by medical device developers due to their ability to meet the pharma and biopharmaceutical industry’s need to take their drugs to clinic and to market at comparatively shorter timelines and lower costs. Despite these advantages, conventional platforms do have some disadvantages. For instance, a clear-cut trade-off for fast development time entwined with platform devices is the loss or diminishing level of flexibility to device customizations, whether it be in accordance with the customer, primary container, branding, or patient requirements. Yet with the rising need for products to offer design differentiation for brand and usability requirements, devices are increasingly expected to offer flexibility beyond the confinements of a platform product.
This is where the addition of modularity is key.
The concept of modularity stems from the general attempt to operationalize and understand complex systems. In a modular platform, the product is divided into modules that can be swapped with other elements of different sizes or functionality to create variants.1 Asset flexibility, cost of goods, and investment deferment are just some of the key drivers behind modular systems.2 When done correctly, modularity supports standardization, repeatability, as well as customization.
In autoinjector development, this translates into the opportunity to leverage all the advantages of a traditional platform device technology while still allowing for various customizations in the device’s design and development. The result is a flexible platform – or a modular platform – that can meet the requirements for industrial design customization and production scaling. In other words, modularity offers double competitiveness to organizations in the form of product cost-effectiveness and freedom of customization.
SHL Medical’s Molly® and Molly® 2.25 mL autoinjectors are built upon a modular platform technology that supports this duality in standardization and customization. First introduced in 2010, the Molly® platform has supported the development and regulatory approval of 17 combination products around the world.3 And it is upon these successes and wealth of experience that Molly®’s modularity is established.
Molly®’s technology is modular in that both the front and rear sub-assemblies comprise intricately designed parts that are configured to allow for an appreciable level of freedom for customization while maintaining its core drug delivery mechanism. For instance, in addition to changing the color of the cap, needle cover, and plunger rod, industrial design customizations in the device’s body and cap are also possible. This allows pharmaceutical companies to introduce unique designs not only for branding and market differentiation, but also for patient distinguishability and usability. Compared to creating a completely bespoke autoinjector, customizing an established platform supports the simplification of project processes and optimization of timelines4.
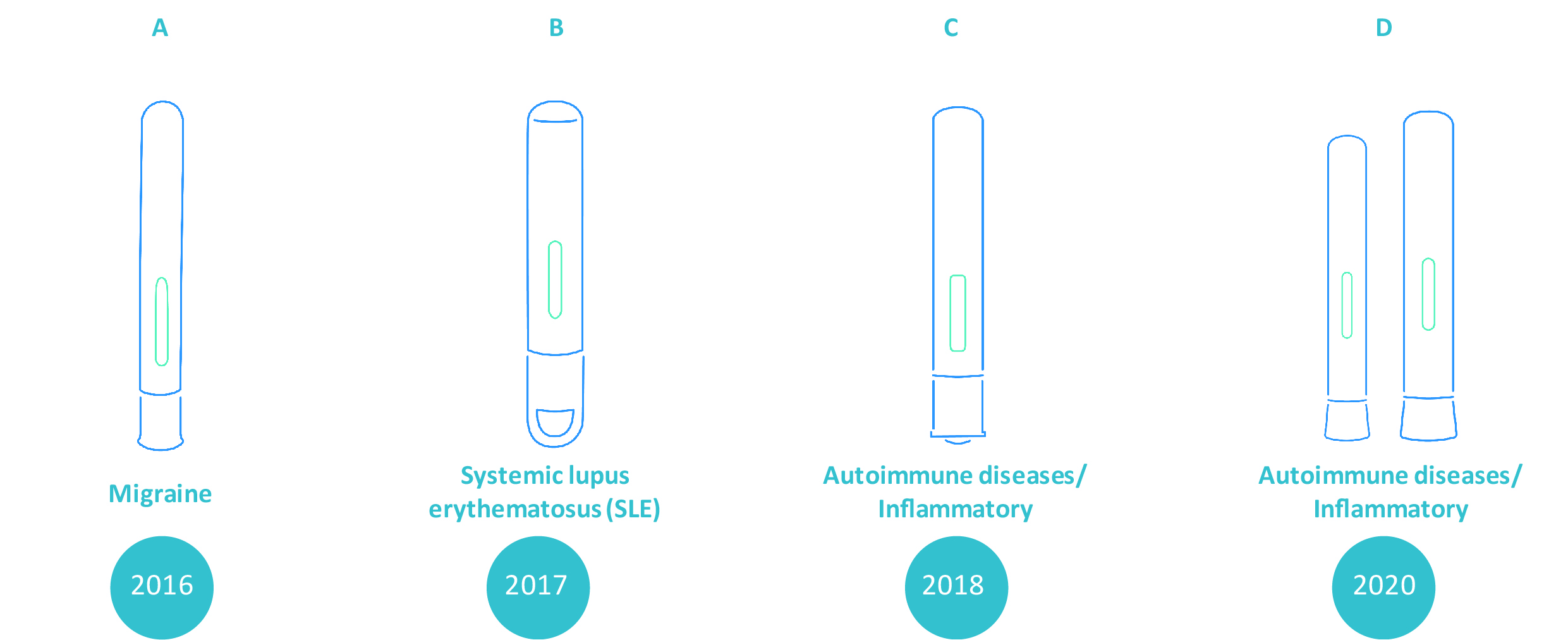
Examples of commercialized Molly® projects with various levels of design customization
For device projects based on the Molly® technology, modularization is not only applied to the device design, but also to the development infrastructures that support it. For example, SHL’s automated assembly and testing equipment platforms also utilize modular infrastructures that can be adjusted to process a mix of different Molly® designs – or several versions of the same Molly® design – at speed, on a flexible scale, and with consistent quality. This complementation ensures that customizations in the device design can be assembled and tested through common machines that feature interchangeable tools and stations corresponding to the device’s characteristics. Moreover, by reusing much of the machinery to assemble and/or test different injection devices, emissions from producing and operating individual pieces of machinery are reduced, increasing the overall sustainability of the final product.
Molly®’s customer-focused model has made it SHL’s most versatile offering to date. Molly® is currently supporting around 14 molecular entities approved for a range of disease areas. This includes one of the world’s first regulatory approved autoinjectors in the high-volume (≥ 2.0 mL) range, as well as a recent combination product addressing obesity. But the true value of Molly® lies in its flexible design and development model that will continue to scale in response to industry advances, such as in data science and future device add-ons that live within a reformulated digital health ecosystem.
For more information on the Molly modular platform technology, please visit the Molly® website
References:
- Zha XF, Sriram RD, “Platform-Based Product Design and Development: A Knowledge-Intensive Support Approach.” Knowledge-Based Systems, vol. 19, no. 7, pp. 524–543, 2006.
- Hernandez R, "Modular Manufacturing Platforms for Biologics". BioPharm International, 28 (5), 2015.
- Internal data as of July 2021.
- Wild L, Fuensalida Pantig GR, “The “Customisable Platform” Paradox”. ONdrugDelivery, Issue 113 (Oct 2020), pp 80–85.

Related News
-
News Pharmapack Awards 2024 Patient-Centric Design Award Winner – Dr Ferrer BioPharma
The 2024 Pharmapack Awards celebrated the best in innovation and design for the pharmaceutical packaging and drug delivery industry on January 24, 2024. -
News Pharmapack Awards 2024 - Celebrating Packaging and Drug Delivery Innovation
The 2024 Pharmapack Innovation Awards ceremony celebrated the best in pharmaceutical packaging and drug delivery innovation at all levels. The awards were held on January 24, 2024 at the Paris Expo Porte de Versailles. -
News Women in Pharma: Looking back on 2023 and moving forward to 2024
In this monthly series, we interview women from across the pharmaceutical industry and supply chain to discuss the importance of gender diversity in healthcare, the workplace, and beyond. -
News On track at CPHI Barcelona - The Track Sponsor interview: USP
In our packed out content sessions at CPHI Barcelona this year we focus on some of the hottest topics coming up in the pharma industry, with each track sponsored by a leading expert in the field. -
News Women in Pharma: Marketing for the other half in healthcare
In our new monthly series, we interview women from across the pharmaceutical industry and supply chain to discuss the importance of gender diversity in healthcare, the workplace, and beyond. This instalment highlights not only the importance of ma... -
News Bringing the pharmaceutical supply chain closer to home
The pharmaceutical supply chain has encountered numerous disruptions in the last few years, impacting procurement, manufacturing, packaging, and distribution operations within the pharmaceutical industry. Read about the rise in calls for near/resh... -
News Delivering on mRNA-based therapeutics: innovations in applications and packaging
Since the onset of the COVID-19 pandemic, the innovative potential of mRNA vaccines and therapeutics has raised questions regarding their manufacturing, packaging, and storage/transportation. Learn about how the pharmaceutical supply chain is meet... -
News Your Prescription for Marketing Success: Digital Pharma Marketing Toolkit – Free eBook
Download your FREE pharma marketing eBook to learn why it is so important for pharmaceutical marketeers to develop their digital content marketing strategies in order to establish their companies as thought leaders and industry experts.
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance