Lyophilisation
Lyophilisation Companies (41)
Lyophilisation News
-
News Lyophilisation technology improving thermal stability and reducing cycle times, says Recipharm expert
Lyophilisation or freeze drying is an important means to improve thermal stability of a drug product, according to Thomas Becker, Quality Director and Qualified Person, Recipharm. This is particularly true for those that are thermolabile, such as mRNA ... -
News Alcami to manufacture PharmaTher’s proprietary ketamine products
The CDMO will supply expertise in GMP sterile fill-finish manufacturing and controlled-substances -
News China SXT's new lyophilisation facility secures pharmaceutical manufacturing permit
The move, coupled with the plants's passing of a pharmaceutical GMP compliance inspection, will enable company to accelerate the R&D of its novel Advanced TCMPs -
News Coriolis Pharma expands facilities for ATMP development
The expansion close to company's Martinsried headquarters includes a lyophilisation development centre for advanced therapy medicinal products
Lyophilisation Products (91)
-
Product Spray Dryer
GEA Niro pharmaceutical spray dryers from R&D to large production scale. Come and discuss your Pharma Spray Drying project with us or learn more about our pharma spray drying technology. Welcome!
-
Product Zeta - Pharmaceutical freeze drying systems
For GMP processes in pharmaceutical applications, we offer the Zeta freeze dryer series featuring the dual-chamber principle. The big integrated intermediate valve separates the two parts: product chamber and ice condenser. The large opening provides ideal flow conditions for water vapour. The dual-chamber...
-
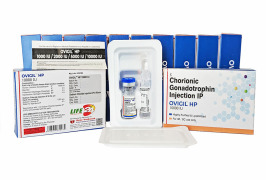
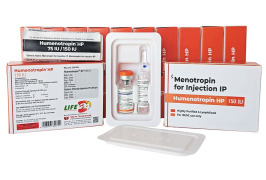
Product HMG/Menotrophin for Injection BP 75 IU -Humenotropin HP 75 IU
The Gonadotropin That Delivers – Precision, Reliability & Consistency
Shree Venkatesh International Ltd offers a wide range of pharmaceutical products, including the Human Menopausal Gonadotrophin 75 IU (lyophilized, sterile powder) & Sodium Chloride Injection BP 0.9% w/v (HMG Inj...
-
Product Formulation of lyophilized products
A correct formulation means greater stability of the active ingredient and provides protection against the stress suffered during the lyophilization process itself.
The selection of the appropriate excipients is really important in the development of a freeze-dried product.
-
Product RheaLyo Mono Freeze-Dryer
The Single Vial Continuous Freeze-Drying System is specifically designed for R&D production based upon the RheaVita Continuous Freeze-Drying Technology. The equipment is perfectly suited for very fast formulation and process development and optimization using a very limited amounts of product by mimick...
-
Product Lyophilized Freeze Drying Tablets (LyoDis / LYOC) - Oral lyophilisate
galien+ Never site is specialized in oro-dispersible forms obtained by freeze-drying process known under LyoDis® LYOC® brand.Nevers site also exports its expertise in Africa and Middle East markets.
What is LyoDis® / LYOC® ?
...
-
Product Freeze drying
The value proposition generated through two sites specialized in freeze drying is unique, from manufacturing process optimization to producing freeze dried characterized and documented vials, allowing our clients to diff erentiate on their own markets. We recently invested 7 million euros to increase capac...
-
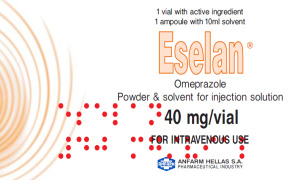
Product NABIQASIM INDUSTRIES / Lyophilization of PPIs / Lyophilized Infusion / Injections / Omeprazole I.V. / Esomeprazole I.V. / Pantoprazole I.V. / Lansoprazole I.V. / Vancomycin I.V. / Colistimethate Sodium I.V.
Lyophilization or freeze drying is a process in which water is removed from a product after it is frozen and placed under a vacuum, allowing the ice to change directly from solid to vapor without passing through a liquid phase. The process consists of three separate, unique, and interdependent processes; f...
-
Product Lyophilisation
Famar offers lyophilisation services. It can perform aseptic filling and lyophilisation in vials from 2ml to 20ml.
Contact us for more information!
-
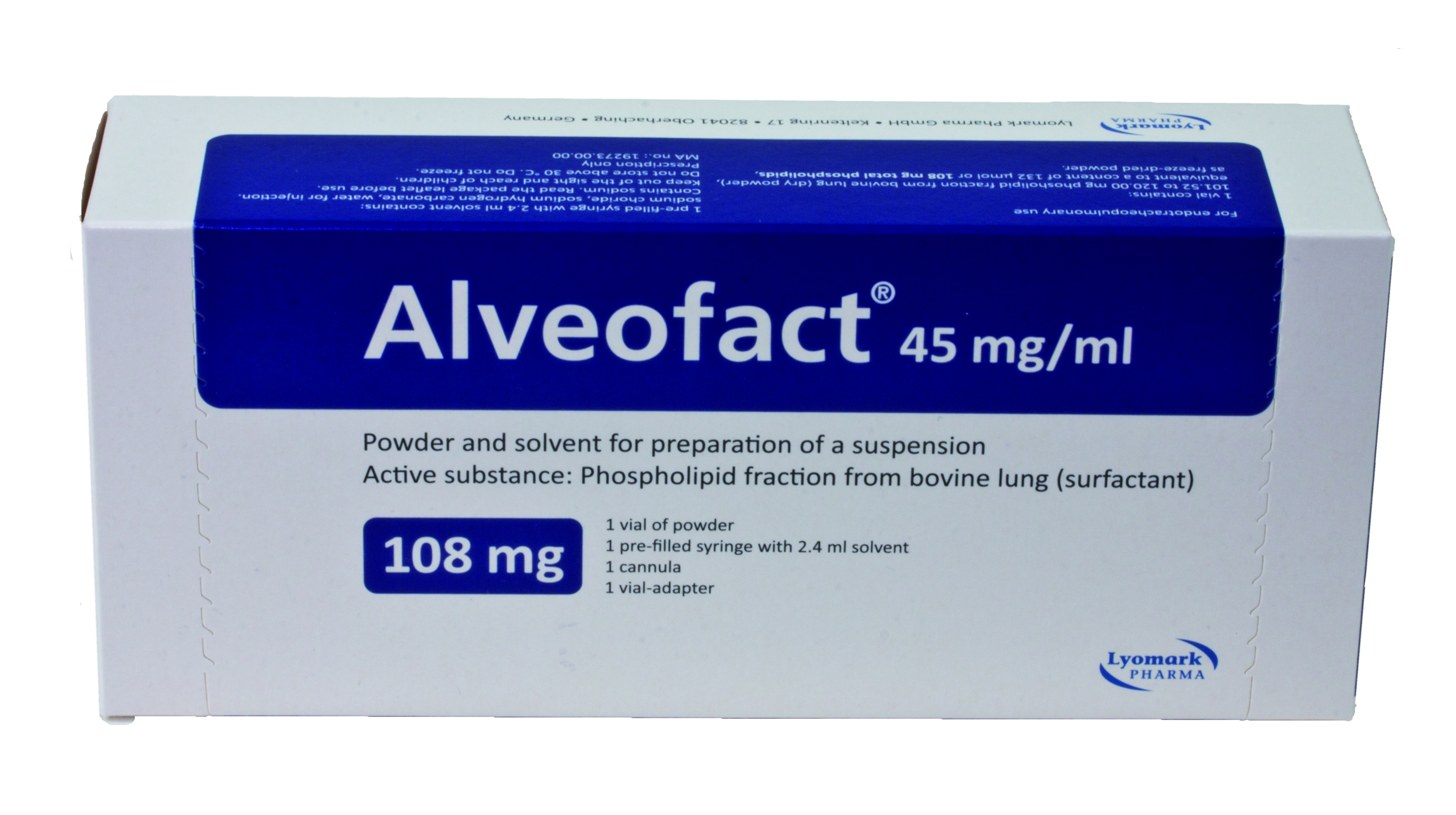
Product CordenPharma Sterile Powder Lyophilized Vials
CordenPharma has extensive experience in sterile contract manufacturing with a wealth of lyophilization expertise. In particular, the optimization of the freeze drying process as it relates to cycle times represents a critical attribute for complex parenteral product development. CordenPharma experts suppo...
-
Product Lyophilization
Mefar offers wide range of products which includes lyophilization in vials with its 20 million + capacity with its state of the art freeze dryers.
Mefar's all services have EMA certification.
Contact us for more information.
-
Product Vitarojal® classic 1000 mg liquid food supplement with royal jelly and honey in vials
Apipharma d.o.o. Offers a wide range of products which includes vitaroja.l 1 vial contains 333.3 mg of lyophilized royaljelly which is the equivalent to 1000 mg offresh royal jelly.Vitarojal® classic 1000 mg contain royaljelly which is known for its biostimulating properties.Each vial contains 1000 mg of r...
-
Product Freeze-drying
Freeze-drying, also known as lyophilisation, is frequently used to develop novel pharmaceuticals, biomaterials and a range of soft and hard tissues. EMCM can offer GMP approved freeze-drying services to its customers for a wide variety of products and production scales.
-
Product Cisplatin
CISPLATIN 50 mg lyophilized vials – Several types of cancer (testicular, ovarian…). Authorized EU-CTD Dossier
-
Product Vigor Forte
Lack of appetite, growth, child’s asthenia in the convalescences of infectious or debilitating diseases.
DOSAGEfrom 2 to 6 years: 1 vial a day preferably in the morning.
INGREDIENTSIn the filler cap: Freeze-dried Royal Jelly 100mg.In the vial: Fresh Royal Jelly 300mg, Acacia hone...
-
Product Aseptic Fill Finish
We offer two convenient fill-finish options for parenteral drug products: prefilled syringe and vials. As a leading pharmaceutical partner, we can provide all necessary elements of delivering high-quality parenteral products for clinical and commercial needs.
AbbVie will leverage its know...
-
Product Pantoprazole Pd.Inj.Sol 40mg/Vial (EU CTD Available)
Pantoprazole 40 mg/Vial Powder for Solution for Injection
Reference Product: Controloc® / Takeda
Description: Pantoprazoleis a proton pump inhibitor indicated for the treatment of the Gastroesophageal Reflux Disease (GERD) with erosive esophagitis (EE) and in Pathological hypersecretion ...
-
Product Dexamethasone Sodium phosphate ®
EIPICO offers a wide range of corticosteroids which includes dexamethasone sodium phosphate ®, dexamethasone sodium as phosphate 8mg /12ml. It belongs to systemic hormones category. Contact us for more information.
-
Product Lyophilization
Developing and optimizing lyo cycles to support clinical and commercial programs
-
Product Sterile suites capabilities
CLINICAL AND COMMERCIAL MANUFACTURING
A UNIQUE MANUFACTURING SYSTEM SPECIALLY DESIGNED FOR ANTICANCER DRUGS, WITH THE FLEXIBILITY TO MANAGE SMALL MOLECULES AND THE COMPLEXITY OF ADCs

Upcoming Events
-
CPHI Middle East 2024
Riyadh Front Exhibition & Convention Center Riyadh, Saudi Arabia
10 Dec 2024 - 12 Dec 2024 -
Pharmapack Europe 2025
Paris Expo, Porte de Versailles - Hall 7.2 | Paris, France
22 Jan 2025 - 23 Jan 2025 -
CPHI Japan 2025
East Halls 4, 5 & 6, Tokyo Big Sight, Tokyo, Japan
09 Apr 2025 – 11 Apr 2025
Pharmaceutical Industry Webinars
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance










































.jpg)



.jpg)
%20(2).jpg)
.jpg)

.jpg)






















































.jpg)




























.png)







.png)

.png)



.jpg)


