Market News
Market news
-
News Apax Partners to acquire the operating subsidiaries of Invent Farma
Invent Farma develops, manufactures and markets generic drugs. -
News Brexit uncertainty favours internationally focused pharmaceutical professionals
Its likely that a number of firms will relocate their services to mainland Europe or Ireland. -
News BioStem Technologies begins construction of new laboratory facility
New facility will house multiple general labs, cleanrooms, and small batch manufacturing rooms. -
News Cambrex completes $50 million investment in large-scale API manufacturing and storage
The company's investment reflects the strong market demand for small molecule APIs. -
News Emulate to collaborate with Seres Therapeutics to support the development of novel microbiome therapeutics
Seres to use Emulate's Intestine-Chip technology to identify promising new microbiome therapeutic candidates for inflammatory bowel disease. -
News SGS introduces fast, accurate genotypic microorganism identification offering at its Chicago, IL facility
FDA guidance states that genotypic methods have been demonstrated to be more accurate and precise than traditional biochemical and phenotypic methods. -
News Cipla to launch South Africa’s first biotech manufacturing facility
The plant will manufacture biosimilars and construction is scheduled to start in early 2017, with full operations expected to commence in the third quarter of 2018. -
News Scots first to receive breakthrough immunotherapy for lung cancer
Eligible patients in Scotland will be the first in the UK to access this life-extending treatment and will be ahead of those patients in England and Wales. -
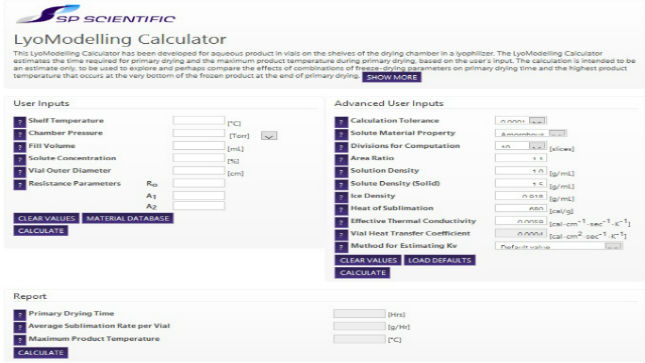
News Powerful software utility for modelling freeze drying cycles
SP Scientific's LyoModelling Calculator enables those developing freeze drying cycles to calculate the time required for primary drying and the maximum product temperature. -
News FDA approves first and only single monthly injection for a PCSK9 inhibitor
Amgen's Repatha (evolocumab) Pushtronex system (on-body infusor with prefilled cartridge) provides a new, monthly single-dose administration option. -
News Global API market revenues to reach $143 billion in 2016
According to a New Market Report Published by Persistence Market Research, the Global API market is estimated to reach $186 billion by 2020, with North America accounting for 35% revenue shares.. -
News New cell culture plate eliminates edge effect during extended incubation
Thermo Scientific Nunc Edge 2.0 plate is designed to cost effectively increase cell consistency and productivity in cell-based assays. -
News CPHI Korea sees 25% growth as domestic pharma economy forecast to become 7th globally by 2020
Government initiatives driving biosimilars and health foods sectors, with increased international partnerships. -
News Claristep filtration system: fast, easy and reliable sample preparation
Fast small-volume clarification that does not add leachables or extractables to the original sample. -
News Shire receives extension of Market Authorization in Europe for Revestive
First therapy indicated in the EU for use in patients aged one year and above with Short Bowel Syndrome, a rare gastrointestinal condition. -
News Major players in type 2 diabetes shifting focus to pricing to maintain market share
With cost pressures burdening many health systems, competitive pricing is a strategy that other companies are likely to adopt with me-too drugs in late-stage development, says GlobalData. -
News Sanofi enters into confidentiality agreement with Medivation
Sanofi agrees to standstill and to withdraw consent solicitation. -
News Sartorius Stedim BioOutsource introduces new Released N-Glycan Assay
In-depth profiling of IgG glycosylation coupled with ADCC assays enables generation of robust biosimilar comparability data. -
News Sartorius Stedim Biotech acquires US start-up kSep Systems
Transaction to expand SSB’s bioprocessing product portfolio with innovative single-use centrifuges. -
News NICE blocks immunotherapy drug nivolumab (Opdivo) for kidney cancer
Decision comes despite nivolumab being accepted for the Early Access to Medicines Scheme (EAMS) based upon some impressive Phase III clinical trial results. -
News RedHill Biopharma and IntelGenx announce definitive agreement for commercialization of Rizaport for migraines with Grupo JUSTE in Spain
Rizaport was recently approved for marketing in Germany under the European Decentralized Procedure; RedHill and IntelGenx continue to work together to secure additional commercialization partners for Rizaport in the US, Europe and other territories. -
News Transdermal Delivery Solutions and MHC Medical Products announce license for Penetran+Plus topical pain reliever
With the acquisition of Penetran-Plus, MHC expands into the OTC pain relief market. -
News Recipharm increases blow fill seal capacity in Kaysersberg, France
The investment will bring the total number of lines to eight, to meet the growing demand of the service. -
News BMS acquires Cormorant Pharmaceuticals
Gains full-rights to Cormorant’s HuMax-IL8 antibody program and lead asset HuMax-IL8 in Phase I/II development. -
News SP Scientific details expanded capabilities in pharmaceutical processing
Company now able to offer a one-source solution for a complete range of pharmaceutical processing equipment. -
News Novo Nordisk invests DKK 400 million in an expanded production plant in Kalundborg
New facilities will provide greater flexibility. -
News Zenith Technologies appoints serialization director
The appointment comes as the pharmaceutical industry faces the challenge of complying with new legal requirements for the authentication and barcoding of licensed drug products. -
News Lyon manufacturing facility returns to the Vectura Group
The facility currently manufactures seven oral products for the Group's partners. -
News Merck and Moderna collaborate to advance novel mRNA-based personalised cancer vaccines with Keytruda
Collaboration combines Merck’s leadership in immuno-oncology with Moderna’s pioneering mRNA vaccine technology and rapid cycle time, small-batch GMP manufacturing capabilities. -
News Rx-360 summary of the WHO Draft Guidelines on Qualification and Validation
In addition to the main guidelines, appendices on validation and qualification are included. -
News Arena Pharmaceuticals announces shift to focus on proprietary clinical stage pipeline
Additional cost reductions implemented to support development program prioritisation. -
News China forecasted as the fastest growing biologics market over the next decade with Chinese NCEs within 5 years
International-domestic pharma partnerships to drive next wave of growth, alongside emerging Chinese multinational CMOs. -
News LEO Pharma enters biologics through strategic partnership with AstraZeneca
The partnership covers potential new medicines for atopic dermatitis and psoriasis. -
News Catalent agrees to commercially supply Palatin Technologies' new bremelanotide pen injector product
Bremelanotide is designed to treat hypoactive sexual desire disorder, the most common form of FSD, in premenopausal women. -
News Chinese authorities issue operating approval to Siegfried Nantong
This significant site addition to Siegfried’s production network strengthens the company’s long-term competitiveness. -
News Torrent Pharma acquires manufacturing unit of Hyderabad-based Glochem Industries
With this acquisition, the company will have three API facilities for the regulated markets. -
News GSK's MenABCWY vaccine set to revolutionize meningococcal disease prevention market
Should the MenABCWY vaccine perform well in upcoming Phase III studies, it has the potential to become market leader, says GlobalData. -
News Innovative first-in-class products comprise 30% of diabetes complications pipeline
New targets need to be identified and converted into improved therapeutic options, particularly in order to develop therapies more aligned with the underlying disease pathophysiology, says GBI Research. -
News Sartorius acquires cell screening specialist IntelliCyt
Unique technology for cell analysis in Biopharma applications. -
News Merck partners with South Korean Institute to enhance vaccine production
Collaboration will improve manufacturing processes to deliver greater yield, allowing higher recovery and providing higher purity vaccines. -
News Novartis adds bispecific antibodies to its growing immuno-oncology portfolio through collaboration and licensing agreement with Xencor
Agreement is the latest in a series of acquisitions and strategic collaborations that have bolstered Novartis' deep and diverse immuno-oncology pipeline. -
News Pfizer invests in a new world-class global biotechnology center in China
State-of-the-art facility will foster the continued development of the biotechnology industry in China, further supporting national healthcare reforms. -
News Catalent launches FastChain service
New service meets the increasing demand for speed and flexibility in global clinical trials. -
News Merck’s acquisition of Afferent Pharmaceuticals provides platform into IPF market
The deal will mean Merck acquires Afferent’s lead candidate, AF-219, which is currently in Phase II trials in IPF patients with persistent cough, says GlobalData. -
News Sanner launches senior-friendly closure for effervescent packaging
The new FOG 27 closure can be opened in about a third of the time — and with 70% less physical effort compared with conventional closures. -
News Sandoz announces plans for five major global biosimilar launches by 2020
Investments of more than USD 1 billion in state of-the-art biomanufacturing facilities, backed by industry-leading capabilities as part of Novartis, positions Sandoz to deliver biosimilars at unprecedented scale. -
News Mylan launches generic Avodart Capsules
Product is indicated as monotherapy for the treatment of symptomatic benign prostatic hyperplasia (BPH) in men with an enlarged prostate to improve symptoms, reduce the risk of acute urinary retention and reduce the risk of the need for BPH-related sur... -
News Cobra Biologics and Alligator Bioscience extend drug development partnership
Project will deliver second cell line for manufacture of immuno-oncology antibody ADC-1015 following ADC-1013 success. -
News Nacimbio launches complex programme for antiretroviral drugs production
Cipla agrees to transfer API manufacturing technology reqired for HIV and Hepatitis C drugs. -
News Novartis' Entresto could prevent or postpone over 28,000 US deaths per year among HFrEF patients
A new analysis published in JAMA Cardiology is the first to quantify the possible impact of Entresto's potential benefit in reducing death. -
News Specialty pharmaceuticals: to compare cost with value, more productivity research is key
A new commentary published in The American Journal of Pharmacy Benefits argues that the value of specialty pharmaceuticals — to patients, employers and society at large — has been left out of the discussion. -
News Tablet press investment at Juniper Pharma Services
Latest addition to the company's suite of tablet manufacturing equipment enables the CDMO to produce single and bi-layer tablets in multiple tooling formats. -
News Recipharm announces global serialisation collaboration
The CDMO has formed a global partnership with Marchesini, SEA Vision and TraceLink to introduce new serialisation capabilities. -
News OPKO’S Rayaldee product receives FDA approval
First drug to use Catalent’s proprietary OptiShell capsule technology to achieve extended release profile. -
News Leukemia has largest pipeline and most first-in-class innovation in hematological cancers space
Of the three major indications within hematological cancer, namely leukemia, lymphoma and myeloma, leukemia has the largest pipeline, with 798 products in active development. -
News CPHI Pharma Awards 2016 opens with four extra categories
Entry submission is now open: the awards bestow recognition on the industry’s most outstanding achievements. -
News Murdo Gordon appointed Executive Vice President and Chief Commercial Officer, BMS
He will lead the company's Worldwide Markets in addition to a new Worldwide Commercialization team. -
News Impax signs definitive agreements to acquire generic products
The agreements include the return to Impax of its rights to its pending abbreviated new drug application for the generic equivalent to Concerta (methylphenidate hydrochloride). -
News Idiopathic pulmonary fibrosis market will grow from $907 million in 2015 to $3.2 billion by 2025
Growth will primarily be driven by increased use of expensive therapies, a rise in diagnosed prevalent cases of IPF, and the launch of two novel therapies, says GlobalData. -
News Kite Pharma opens state-of-the-art T-cell therapy manufacturing facility
43,500-sq-ft plant next to Los Angeles International Airport will support production of engineered CAR and TCR cancer immunotherapies. -
News SGS introduces new comprehensive drug compatibility study testing at expanded Shanghai facility
New service offers analysis of potential extractable and leachable contamination within pharmaceutical products. -
News PaizaBio: China approves Drug Marketing Authorization Holder pilot plan
Move approves the use of contract manufacturing organisations. -
News Famar to acquire Roche's Leganes site
Site provides Famar with capacities and expertise in the production of solid dosage forms with high-potency APIs, which represents new technology for the company. -
News ARIAD completes strategic review and announces plans for growth
Key outcome of the strategic review is a commitment to commercializing brigatinib in the US, subject to approval by the FDA. -
News Pharmaceutical industry licensing deals soared to record $46.2 billion in 2015
Pharmaceutical licensing deal values increased by 37.1% year-to-year, from $33.7 billion in 2014 to $46.2 billion in 2015, driven primarily by Sanofi. -
News Breakthrough combination for patients with skin cancer receives fast-tracked ‘yes’ from NICE
This represents one of the fastest ever approvals by NICE – UK patients will be the first to benefit across Europe. -
News NICE denies NHS patients access to Vertex's Orkambi
“As a result of applying the wrong appraisal process, approximately 2,700 people in England who could benefit from Orkambi are being forced to continue waiting for access.” -
News Zika vaccine race overlooks need for global strategy to combat mosquito-borne diseases
Vast majority of Zika vaccine products are currently in early preclinical trials, indicating that it will take many years until one of them receives market approval, says GlobalData. -
News Forte Pharma, Reig Jofre’s nutritional supplements line, enters in Hong Kong and Macao
This operation is part of Reig Jofre's internationalization plan, by which 60% of company sales are now made outside Spain, with a target of 70% in 2019. -
News Bavarian Nordic announces expansion of Imvamune orders from Canadian government
PHAC orders a total of 360,000 doses of the smallpox vaccine. -
News Hovione announces the groundbreaking expansion plans of its New Jersey Facility
Expansion will introduce a new commercial spray dryer unit to complement the existing pilot unit and this installation will be specifically designed to handle potent APIs -
News 80% of pharma companies have new product planning groups on brand commercialization committees during early-stage development
Internal functions are key for companies' committees across stages of development. -
News Sartorius Stedim Biotech launches innovative ambr 250 modular bioreactor system
New expandable benchtop workstation with unique single-use bioreactor design offers a simple approach to process development for fermentation and cell culture. -
News Hyphenated technique reveals valuable information about plasma proteins
Researchers show how an AF4-ICP-MS system is able to obtain valuable information on the multi-element speciation and absolute molecular mass of human plasma proteins such as Albumin and Transferrin. -
News Turkish pharma grows by 15.6%, as CPHI Istanbul shares MENA trends and challenges for 2016
Next 5 years will see more outsourcing, vaccine, biotech development and harmonisation in MENA region. -
News Brexit would irreparably damage UK pharma industry
A Brexit in the upcoming EU referendum could directly damage the UK pharmaceutical industry, according to NonStop Recruitment. -
News New Sartorius Stedim BioOutsource microsite goes live
Cellca’s cell line and upstream process development services now integrated. -
News ASCO 2016: Roche’s latest Tecentriq results underline potential to dominate bladder cancer market
The launch of Roche’s recently-approved PD-1 immune checkpoint inhibitor Tecentriq will usher in a dramatic change in the treatment paradigm of metastatic bladder cancer, says GlbalData. -
News Pharmaceutical companies increasingly looking to innovate high-risk, first-in-class products
'Imperatives for pharmaceutical companies include reducing product development costs, maximizing the annual product revenue, and optimizing the life cycle of a drug,' says GBI Research. -
News Sartorius Stedim Biotech presents Virosart Media
Offers a fast and cost-effective solution for manufacturers to reduce the risk of virus contamination resulting from raw materials during fermentation. -
News Deacom's AutoFinisher automates serialized and catch weight production
New AutoFinisher moves manufacturing jobs from scale to label in under one second. -
News Dr Reddy’s to acquire product portfolio from Teva for US Market
The portfolio being acquired is a mix of filed ANDAs pending approval and an approved ANDA. -
News Merck to acquire Afferent Pharmaceuticals
Afferent Pharmaceuticals is a leader in the development of therapeutic candidates targeting the P2X3 receptor for the treatment of common, poorly-managed, neurogenic conditions. -
News Integrated DNA Technologies acquires oligonucleotide synthesis company ValueGene
Company welcomes new customers with promise to provide seamless support for genomics research. -
News Lonza and bluebird bio establish a long-term commercial manufacturing agreement for Lenti-D and LentiGlobin drug products
Agreement provides bluebird bio with a path to commercial supply including dedicated production suites within Lonza’s state-of-the-art facility, which is currently under construction. -
News AstraZeneca enters commercialisation agreement with Aspen for anaesthetic medicines portfolio
Agreement expands commercial reach for anaesthetics and supports the Company’s sharp focus on innovative new medicines in its three main therapy areas. -
News Merck expands its Investment Fund Merck Ventures
Total fund size doubled to up to €300 million. -
News Alvotech opens state-of-the-art biosimilar facility in Iceland
The new facility will significantly increase Alvotech’s production capacity enabling the group to produce higher yields at lower costs. -
News Disruptive technologies and emerging trends drive life science transformation
New business models and behavioural shifts create opportunities for growth. -
News Sun Pharma divests US manufacturing units
Divest two oral solid dosage manufacturing facilities to Frontida BioPharm. -
News Cobra Biologics and Touchlight collaborate on production of next generation DNA constructs
The collaboration will optimise the manufacture of AAV vectors to support gene therapy and regenerative medicine. -
News Meningococcal vaccines market set to approach $1.8 billion by 2025
The provision of a single vaccine that can protect the population against all prevalent serogroups is the most important opportunity for companies hoping to access a large market share, says GlobalData. -
News Merck explores new risks for biopharma with the EIU
New approaches for mitigating risk associated with new growth strategies. -
News NovaBiotics highlights importance of a co-ordinated approach to antimicrobial resistance
New multi-action, multi-strain anti-infectives needed to win battle against AMR. -
News Catalent opens new clinical supply facility in its Japan campus with studies starting this month
The site will serve the needs of both domestic and global clinical trial sponsors. -
News SGS introduces new hydrogen deuterium exchange capabilities for protein characterisation and biopharmaceutical development
Company will be one of the first service providers to offer HDX-MS in a cGMP environment. -
News Fujifilm Diosynth collaborates with MSD on a new 20,000-L microbial biologics facility
Enables Fujifilm Diosynth’s customers to benefit from MSD’s strong track record with large-scale microbial operations. -
News Hovione opens a new sales and customer support office in Japan
Japan is a strategic market for Hovione where the company is growing both its off-patent APIs and its contract manufacturing businesses. -
News Experts analyse growth in the finished dosage market ahead of CPHI Worldwide launch
New drug delivery and bioavailability technology driving growth globally. -
News Mylan launches generic Cleocin solution
Product is indicated for the treatment of serious infections caused by susceptible anaerobic bacteria and susceptible strains of streptococci, pneumococci and staphylococci. -
News Shire completes combination with Baxalta
Combined company expected to deliver double-digit compound annual top-line growth with over $20 billion in annual revenues by 2020. -
News Particle Sciences adds sterile powder filling and capsule filling capabilities
Powder fills from 50 mg to 75 g, with an accuracy of up to +/- 1% of fill volume, are now possible, including those for highly potent compounds. -
News New merger between Pfizer and Anacor set to leverage companies in atopic dermatitis space
While Anacor’s crisaborole will benefit from Pfizer in terms of marketing power, its release will subsequently pave the way for Pfizer to launch its own candidate, a topical form of Xeljanz, says GlobalData analyst. -
News Global pharmaceutical firms start prioritising patients over pricing
GSK puts drug affordability before IP protection in low-income countries. -
News Carbogen Amcis expands operations in Bubendorf
The acquired multi-story building is divided into laboratory, production, storage and office areas and provides a set of cleanroom GMP-compliant suites. -
News CMC Biologics expands GMP manufacturing capacity in Europe
Addition of a Bioreactor 3PACK production facility to its Copenhagen facility provides additional flexible solutions for clients. -
News WuXi Biologics and Sartorius Stedim Biotech open joint laboratory in Shanghai
Certified leading upstream platform powers China’s biopharmaceutical development. -
News New data from trials of Opdivo support its efficacy in recurrent metastatic renal cell carcinoma
The drug has been hailed as new standard of care in mRCC due to promising survival outcome rates and objective response rates, say GlobalData analysts. -
News Pharmapack Europe launches new Start-up Hub
All-new addition for 2017 designed to let innovative young pharma firms benefit from valuable networking and learning opportunities -
News Horizon Discovery Group’s molecular reference standards integrated into QIAGEN GeneReader NGS System
Deal demonstrates the impact Horizon's Reference Standards are having in supporting the rapidly growing Next Generation Sequencing (NGS) market. -
News I Holland showcases its new tooling products at Interphex Japan
Tooling products focused on increasing productivity for the tablet manufacturer. -
News Shire shareholders vote to approve combination with Baxalta
Combination would create the global leader in rare diseases. -
News Xellia Pharmaceuticals expands Budapest facilities to strengthen anti-infective product capabilities
Foundation Stone Laying Ceremony of new $10 million Centralized Laboratory Services building attended by Deputy State Secretary Kristóf Altusz from the Hungarian Ministry of Foreign Affairs and Trade, and Mayor Mr Róbert Kovács -
News DCGI gives limited approval to market FIRST Allogeneic cell therapy product in India
Stempeutics puts India on the 'World map of Regenerative Medicine'. -
News Catalent strengthens leadership position in Asia-Pacific
Company announces key appointments and a new sales office in Korea. -
News New research from Accenture reveals gap in Pharma R&D’s journey to delivering better patient outcomes
Adoption of digital transformation to drive patient outcomes may improve competitive position for pharma R&D organizations. -
News Circadian Design now taking pre-orders for Round Refill medication delivery service with smart bottle
Targets $289 billion annual cost to US healthcare system. -
News Colorcon acquires Kollicot Coating Systems line from BASF
Company also advances co-operation to offer superior functional coatings for pharmaceutical tablets. -
News Tablet development made easy
Gemlen tableting launches series of videos discussing the key principles of tableting.
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance







.jpg)