Market News
Market news
-
News API supply chain needs to address future challenges arising from China situation: CPHI Worldwide panel
COVID-19 pandemic has exposed weaknesses and sector faces new challenges driven by rising costs of production in China -
News Digitalisation the key to efficient EU pharmaceutical regulation and easing of localised drug shortages
Current EU regulatory systems too overwhelmed by paper, head of European association representing generics, biosimilars and value added medicines industries tells CPHI Worldwide online conference -
News Indian pharma industry on cusp of growth spurt, CPHI Worldwide audience told
Country expected to grow threefold over the next decade due to increased focus on complex generics, biosimilars and specialty products -
News GTP Bioways to galvanise CDMO position in France with two new biopharma facilities
One site will focus on microbial production, the other on small-scale mammalian-based production -
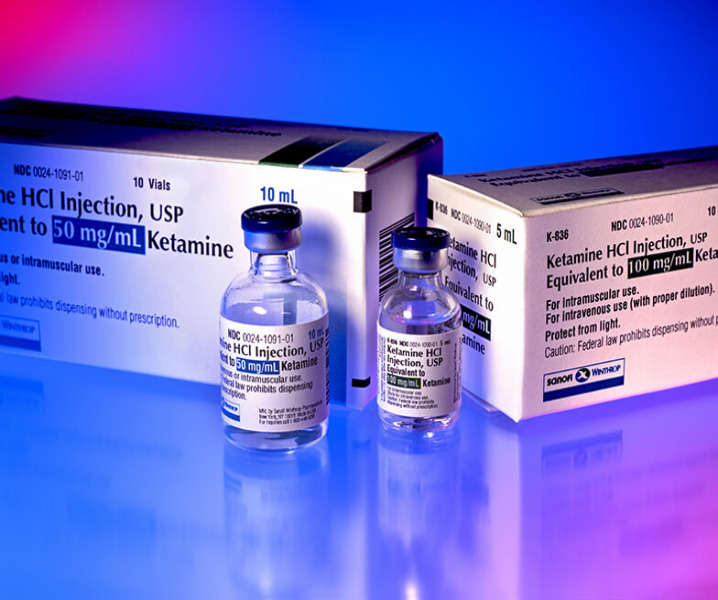
News Alcami to manufacture PharmaTher’s proprietary ketamine products
The CDMO will supply expertise in GMP sterile fill-finish manufacturing and controlled-substances -
News Phlow receives approval to join BARDA CDMO network
As a network member, the company hopes to help reduce the US's reliance on overseas imports by providing domestic API capability -
News Novavax defends COVID-19 vaccine manufacturing processes after claims of quality issues
According to unnamed sources in Politico report, company’s vaccine batches have not met FDA purity level standards -
News FDA misses approval target dates after plant inspections delayed by COVID travel restrictions
UCB Pharma is informed by agency that action on application for psoriasis treatment is being deferred until on-site visits can be made -
News Matica Bio and Sartorius form large-scale vector production research pact
Together the companies will attempt to overcome "black box" challenges to enable more robust and consistent results for clients -
News China SXT's new lyophilisation facility secures pharmaceutical manufacturing permit
The move, coupled with the plants's passing of a pharmaceutical GMP compliance inspection, will enable company to accelerate the R&D of its novel Advanced TCMPs -
News Avid Bioservices to plug capacity gap with new viral vector manufacturing facility
The biologics CDMO says it expects its new 53,000-sq.ft facility will be operational within 18 months -
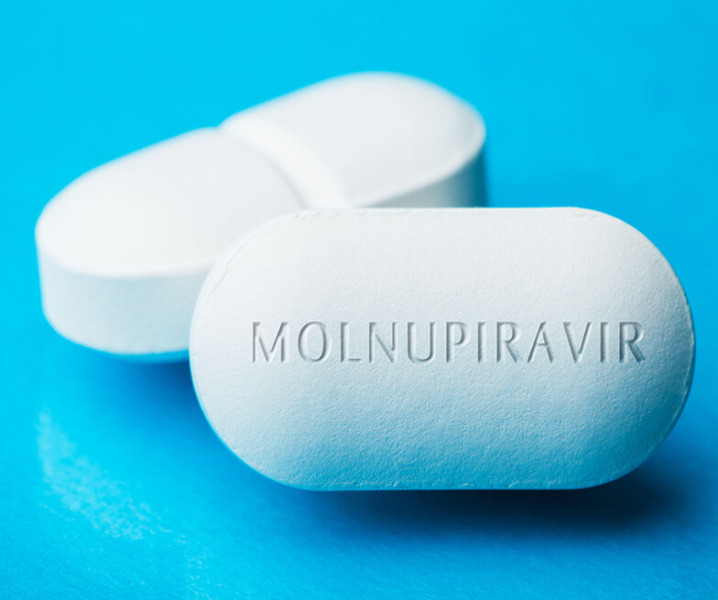
News Everest Organics starts API production for Merck anti-COVID pill
Manufacturer starts development of API for molnupiravir, which is set to become world’s first oral treatment against coronavirus -
News Pharmapack 2021: Barriers to healthcare adoption of growing sustainable packaging trend still exist, say experts
Panel discusses how healthcare institutions are adapting to calls for more sustainability in various processes in the value chain -
News Aceto boosts biomanufacturing footprint with acquisition of Ireland-based A&C Bio Buffer
The transaction expands the CDMO's GMP biopharmaceutical and vaccine manufacturing capabilities -
News Merck KGaA doubles gene therapy capacity with opening of second Carlsbad CDMO facility
The new the 140,000-sq. ft facility will support commercialisation and industrialisation of viral vectors -
News Eckert & Ziegler expands CDMO services to support radiopharma companies
Having acquired a 53,000 sq-ft property in Berlin, the company plans to set up new laboratories, production and cleanrooms -
News Pharmapack 2021: Pre-filled syringe technology could provide answers to future mass vaccination challenges, says BD
Pre-filled syringes (PFS) could help immensely in solving the challenges of rolling out future mass vaccination campaigns, according to experts at Becton Dickinson (BD). -
News Boehringer opens doors to new Austrian biopharma production site
The LSCC provides an additional 30% in mammalian large-scale capacity for the company's own pipeline and for third parties -
News COVID-19 triggers growing interest of European patients in where drugs are manufactured: Teva study
Just as 'food miles' have become a key concern among consumers in recent years, patients now want to know more about 'medicine miles', study commissioned by Teva finds -
News Pharmapack 2021: Convergence of trends are pushing drug delivery devices towards connectivity, says Nemera
Electronic devices and connectivity are being progressively included in drug delivery devices, bringing positive outcomes to all stakeholders, according to drug device combinations specialist, Nemera Tuesday -
News Forge Biologics to provide CDMO support for Solid Biosciences' preclinical gene therapy
By providing AAV process development, scale up and cGMP manufacturing services, Forge hopes to bolster Solid's gene therapy pipeline -
News MannKind signs sale-leaseback agreement for inhaled insulin manufacturing site
The transaction will generate $102.25 million to support the company's product pipeline and scaling up commercial activities for Afrezza -
News Fujifilm begins commissioning phase of new Netherlands-based manufacturing facility
The facility will increase the production capacity of cell culture media products and provide a hub for European and global markets -
News Sartorius to add flagship site in North America
The company plans to open a new 130,000-sq. ft plant in Ann Arbor in late 2023 -
News Hikma boosts US injectables business with $425 million deal for Custopharm
The acquisition will add 13 approved products and additional pipeline products -
News Report accuses Covid vaccine makers of putting profits before lives
Only 0.3% of the 5.76 billion doses administered worldwide have been distributed to low-income countries -
News SK Capital Partners in discussions to create an integrated global API CDMO leader
The private investment firm plans to acquire SEQENS and merge it with Wavelength Pharmaceuticals -
News Fresenius Kabi invests in Pre-Filled Syringe Capacities for US and EU supply
As a safe, effective and convenient way to administer injectable medications, pre-filled syringes (PFS) have become the injection system of choice for a growing number of drugs. PFS allow healthcare professionals to easily handle syringes, reduce waste... -
News New Thermo system makes AAV production more efficient and scalable
The company's new Gibco AAV-MAX Helper Free AAV Production System helps reduce production costs and streamline transition from research to clinical environments -
News Catalent and Phathom Pharmaceuticals strike commercial supply agreement
The CDMO will provide commercial manufacturing and packaging services for Phathom's novel, orally active P-CAB -
News Catalent boosts nutraceuticals market presence with $1 billion Bettera acquisition
The transaction is expected to accelerate Catalent's growth in global softgel and oral dose formulation and manufacturing services -
News Clean Cells to invest €13 million to create Europe's largest cell bank production site
The expansion is anticipated to speed up development and time-to-market of novel treatments and vaccines for COVID‑19 -
News AGC Biologics to increase capacity for pDNA and mRNA manufacturing in Heidelberg
The expansion plans include an additional manufacturing line, a new cleanroom and a new process development lab -
News Room for specialists and one-stop-shops in CDMO space
As CDMOs seek to diversify their business models, is a full-service offering the holy grail or is there a danger of biopharmaceutical outsourcing firms becoming the jack of all trades and the master of none? -
News Thermo Fisher to build facility in Nashville for production of single-use technologies products
Investment will further expand CDMO's global network of SUT facilities to boost reliable supply of critical materials used to produce new biologics and vaccines -
News Emerging regional markets show promise as CDMO globalisation trend grows
Opportunities for CDMO expansion are extending to Latin America and Africa, say experts -
News GenScript ProBio to manufacture clinical trial batches of Synimmune's lead antibody drug
The CDMO will manufacture drug substance and drug product for Phase II clinical trials in AML patients -
News Takeda to supply Japan with 150 million doses of Novavax COVID vaccine
Japan's biggest drugmaker prepares to make the vaccine domestically and anticipates early 2022 distribution -
News Formulated Solutions completes investment and expansion project
The CDMO significantly increases its footprint, capacity and capabilities to meet current and future demand -
News 3i Group backs ten23 health to create global CDMO focused on biopharma injectables
The new Switzerland-based CDMO differentiates itself by its sustainable vision of making a positive impact for people and planet -
News Reopened USAntibiotics penicillin facility to relieve US dependence on Chinese antibiotics: Jackson Healthcare
The facility will be able to manufacture and stockpile a five-year supply of amoxicillin for the US -
News Cipla and Kemwell to form joint venture targeting respiratory biosimilars market
The agreement is expected to accelerate Cipla’s global lung leadership agenda -
News Medicines Manufacturing Innovation Centre aims to 'solve major pharma industry challenges'
£35 million public-private collaboration to develop next-generation pharmaceutical manufacturing processes to drive forward innovation in the medicines supply chain -
News Recipharm expands its analytical services offering in India
The CDMO inaugurates its new analytical laboratory under Recipharm Analytical Solutions -
News Signature Biologics opens additional manufacturing and R&D facility
The new facility will augment the company's capacity to broaden its portfolio of perinatal-derived products -
News Lonza to establish multi-product fill and finish line in China
The new production line at Guangzhou site will supply global and domestic companies with clinical and commercial batches -
News Curia lays out plans to expand complex API manufacturing capacity
The $35 million investment will more than double the site’s batch-size scaling and product output. -
News Biotage expands production capacity to ease lipid bottlenecks
New site in Cardiff boosts the company's production capacity for equipment crucial in the supply chain for mRNA vaccines production -
News Tikun Olam-Cannbit and Wavelength strike novel cannabis agreement
Firms say the partnership will "multiply the power of both companies, the pharmaceutical industry and the medical cannabis industry" -
News Recro eyes expansion to full-service CDMO status
The solid oral dose specialist signs letter of intent for acquisition of a CDMO providing a full range of services -
News Moderna to build mRNA vaccine manufacturing facility in Canada
The new facility will provide access to domestically manufactured vaccines against respiratory viruses -
News 5 Things We Learned from the CPHI North America Content Program
The CPHI North America exhibition is taking place in-person this week in Philadelphia from 10-12 August. Here we take a look at key learnings from the past two weeks of expert-led content, available to all attendees as part of the hybrid event experien... -
News Bora Pharmaceuticals and KYOWA agree generic manufacturing contract
KYOWA Pharmaceutical Industry will be the CDMO's first Japanese client for its Zhunan manufacturing site -
News Cosmetics packaging needs to respond to increasing consumer awareness of sustainability: CPHI Webinar panel
The cosmetics packaging sector will need to respond to increasing consumer awareness of climate change by transitioning from fossil-based materials to long-term carbon neutral and carbon negative alternatives, according to industry experts speaking at ... -
News Tech transfer: a many-layered artform that demands precision and communication
Tech transfer is a crucial phase that converts clinical promise into commercial gain but pressure on sponsors to reduce time to market in the age of COVID-19 means CDMOs have to be on top of their game to deliver. What are the latest innovations to sol... -
News DisperSol signs up Catalent for manufacturing of multiple pharma products
The collaboration will see Catalent install a commercial-scale KinetiSol technology manufacturing line at its Somerset, New Jersey, facility -
News WuXi STA completes acquisition of its first European facility
The purchase of BMS's Couvet, Switzerland facility adds commercial-scale production capacity for capsule and tablet dosage forms -
News Viatris and Biocon score historic first for interchangeable biosimilar product
The new approval of Semeglee allows pharmacy-level substitution for Lantus across the US -
News Diversification and addition of capabilities key to CDMO success as end markets grow: CPHI North America panel
CDMOs need to diversify and expand their capabilities to take full advantage of strong growth in prominent end markets such as small molecule, monoclonal antibodies and cell and gene therapy, according to an industry expert on a panel at the CPHI North... -
News Sartorius extends its cell culture media business via acquisition
Acquiring Xell AG will enable Sartorius to offer specialised media for manufacturing viral vectors -
News Thermo Fisher pledges to reach carbon neutrality by 2050
Accelerated efforts align with the Paris Agreement and Race To Zero to combat climate change -
News Abzena and BioXpress Therapeutics partner to provide global biosimilar support
The combined capabilities will offer flexibility and innovation from small-scale biosimilar development through to large-scale manufacturing -
News Merck KGaA invests €270 million to create "site of the future"
The investment will fund the construction of a Translational Science Center for its Healthcare business sector, as well as a new Learning Center -
News BioNTech to purchase Kite manufacturing facility and cell therapy R&D platform
The acquisition will add manufacturing footprint in the US enabling the company to develop its pipeline of novel cancer product candidates -
News Samsung Biologics and Kineta strike cancer immunotherapy development and manufacturing deal
Samsung to provide end-to-end CDMO services to advance Kineta's novel anti-VISTA antibody for the treatment of solid tumours -
News Skyepharma selects Veratrak platform to ensure secure data storing and sharing
Using Veratrak's platform, the CDMO will have full oversight of data, including an audit trail of data and document processes -
News Curia to buy fellow CDMO Integrity Bio
Acquisition will add West Coast coverage to the CDMO's East Coast and European capabilities and enhance its biologics formulation development and fill-finish network -
News Catalent enters CBD anaesthetic product formulation project
The company will use its orally disintegrating tablet technology Zydis for JOS Pharmaceuticals' licensed CBD product -
News Sparx Therapeutics to build first commercial antibody drug production facility in China
The emerging biotech company unveils plans for a 1.2 million-sq. ft manufacturing facility in Yangzhou -
News LGM Pharma launches analytical and stability testing as standalone service
The integrated CDMO rolls out services to the broader community of drug manufacturers and developers -
News Thermo Fisher supports gene developers with new AAV manufacturing solutions
A new media panel, gene kit and advanced resins help reduce manufacturing costs and increase the viability of gene therapies -
News Novartis shifts Leqvio manufacturing to Austrian facility
Pharma firm tells FDA tech transfer to its own facility in Schaftenau, Austria is underway after regulator highlighted concerns about third party CDMO site in Italy -
News Recipharm selected to operate new fill/finish facility in Morocco
Move is part of US$500 million five-year investment programme by Moroccan government and consortium to establish vaccine and biotherapeutic manufacturing capacity in the country -
News Second Curaleaf GMP cannabis facility gains Spanish approval
Medalchemy facility now fully licensed to import, cultivate, extract, manufacture and export medical cannabis flower and extracts -
News Viralgen opens expanded AAV manufacturing facility in Spain
The CDMO's fivefold capacity increase will provide early preclinical through to commercialisation support -
News Amgen to build new assembly and final packaging facility in Ohio
Greenfield construction of the new facility is expected to start later this year -
News Sanofi to accelerate mRNA vaccines through new Center of Excellence
The company will invest €400 million per year to advance mRNA technology beyond the pandemic -
News Lonza to invest in mid-scale API manufacturing expansion at Chinese facility
The expansion of mid-scale capacity will enable the company to offer a smooth transition between early-phase and large-scale commercial production -
News CPHI Podcast Series: M&A strategies in the CDMO sector
This month we discuss what is driving M&A strategies in the pharma space, paying particular attention to how investors regard the potential for the growing but fragmented CDMO sector -
News Genezen begins construction of cGMP lentiviral production facility
The investment will provide capacity to support current and future demands for the rapidly evolving cell and gene therapy sector -
News CPHI Webinar: Trends and Advances in Patient Centric Drug Development - Watch On Demand
Catch up on the latest trends and developments in patient centric drug development -
News Vectura to provide preclinical support for Incannex's inhaled brain injury treatment
IHL-216A may make sports safer and reduce the morbidity and mortality rates of people suffering serious head traumas -
News ProBioGen and LAVA Therapeutics sign cell line development agreement
The agreement marks the second cell line development collaboration between the two companies -
News Coriolis Pharma expands facilities for ATMP development
The expansion close to company's Martinsried headquarters includes a lyophilisation development centre for advanced therapy medicinal products -
News Pandemic creates extra demand for CDMO services but investment decisions remain key to success
While the COVID-19 pandemic has created even greater demand for partnerships, CDMOs need to be aware that the needle has shifted, creating both opportunities and challenges and changing how the services industry does business -
News Bayer makes €250 million manufacturing investment in Finland
The company expects to complete its building and modernising plans in Turku by 2025 -
News Robust sales growth at Indian pharma companies expected in current FY on pandemic recovery, says Fitch
Drug categories impacted by travel restrictions expected to see higher sales levels in FY22 -
News Construction underway for Yposkesi's second commercial bioproduction site
The French CDMO is also creating a new global resource for drug developers of ATMPs -
News WuXi XDC and LegoChem sign MoD to make more ADCs accessible to patients
XDC will provide a full range of development and manufacturing services in one centralised region -
News Entegris to expand 3 life sciences manufacturing facilities
The expanded capacity and capabilities include the development of a dedicated Life Sciences Technology Center -
News EmpowerPharm certified to manufacture CBD products to prescription drug standards in Canadian first
New $30 million manufacturing facility in Burlington, Ontario, will allow company to manage R&D in-house and scale production without reliance on third-party manufacturers -
News Pharma supply chain companies form alliance to accelerate sustainability
Alliance to Zero aims to facilitate the transition of the pharma sector to reach net zero emissions target -
News Siegfried starts to ramp up production after cyber attack
Hameln plant which provides fill and finish for Pfizer-BioNTech COVID-19 shot should be fully operational within the week, CDMO says -
News MedPharm expands topical and transdermal delivery services
A new location in Raleigh-Durham, North Carolina, close to the CDMO's Center of Excellence, will double its current footprint -
News Bacthera secures double nod to tread on "virgin territory"
The specialist CDMO receives manufacturing licenses from Switzerland and Denmark to supply customers with live biotherapeutic medicines -
News Sharp makes land acquisition to expand cGMP packaging capacity
The purchase is part of $17 million capacity expansion, which is in addition to a wider $43 million investment into the company's Pennsylvania operations -
News ‘Conscious consumer’ demand is propelling cosmeceuticals industry, experts tell CPHI Discover audience
Upstream raw materials suppliers seen as the main source of innovation in a growing industry driven by demand for more natural ingredients manufactured in clean, sustainable way -
News Innovating in the viscosity design space with a new 2.25 mL autoinjector platform
Extending its patient-centric BD Intevia™ Disposable Autoinjector platform, BD builds on past successes while meeting the requirements of new, highly viscous, high-volume biologics for chronic diseases -
News Brexit impact on UK pharma industry a moveable feast, industry experts tell CPHI Discover
The UK’s withdrawal from the European Union has meant the pharmaceutical industry has had to adapt to some monumental changes in terms of research, regulatory affairs, and trade. In this CPHI Webinar, three industry experts discussed what the fut... -
News BD chooses Spain as home for new high-tech manufacturing facility
Zaragoza plant will produce devices such as prefillable syringes to deliver vaccines and other biologics -
News Europe approves SK Bioscience’s global COVID-19 vaccine plant
CDMO's site becomes the first vaccine production facility in South Korea to obtain EU GMP certification, allowing it to ship to Europe -
News Sterling Pharma Solutions signs up to manufacture OncoTEX's ovarian cancer drug
The CDMO's expertise and capabilities a "perfect fit" for overcoming the challenges of manufacturing this complex molecule -
News Catalent buys Belgian CDMO to boost pDNA manufacturing and service offering
New facility at Gosselies site will host commercial scale pDNA manufacturing as Catalent extends its cell and gene therapy footprint -
News CPHI Discover: Earlier partnerships between pharma and excipient manufacturers are the key to success
In the future pharma companies will need to work closer with excipient companies if we are to develop better formulations more quickly -
News Richter-Helm invests €70M to triple its production capacity in Germany
The addition of two new manufacturing trains will support the demand for high-value biopharmaceutical products -
News Ex-Legacy Pharmaceuticals machinery and equipment up for sale, says Hilco Industrial
Industrial assets purchaser is offering high-capacity production line equipment formerly owned by bankrupt CMO -
News Moderna to transform Massachusetts site into industrial technology centre
The expansion will support a 50% increase in production of biotech's COVID-19 vaccine at its manufacturing site -
News N-SIDE launches Production App to optimise clinical manufacturing process
The software consulting company claims its software solution has already saved one pharma company €10.5 million -
News Moderna signs agreement with Gavi to supply COVID-19 vaccine
Biotech ties up deal three days after World Health Organization issued an emergency use listing for the mRNA vaccine -
News Alibaba bans online listing and trade of pharmaceutical products
Sweeping ban includes APIs, intermediates and prescription and OTC medicines and comes amid tight regulatory requirements and the "high risk nature" of pharma products, e-commerce giant says -
News Bushu and Suzuken team up to support manufacturers eyeing up Japanese market
The new business alliance will support new product launches for specialty pharmaceutical manufacturers looking to enter the Japanese market -
News Sanofi to provide fill and finish manufacturing support for Moderna vaccine
French company is now providing manufacturing capacity to support three different COVID-19 vaccines -
News Lonza to build small molecule manufacturing complex at Visp site
CHF 200 million investment will include dedicated line for antibody-drug conjugate payload molecules and is scheduled to start operations in Q3 2023 -
News FDA inspection report slams Emergent for quality and cleanliness breaches at Bayview facility
Company failed to investigate vaccine drug substance discrepancies thoroughly, says US regulator -
News Bharat Biotech scales up Covaxin manufacturing capacity in India
The company will expand multiple facilities in Hyderabad and Bangalore to help reach its target of approximately 700 million doses per year of the COVID-19 vaccine -
News AGC Biologics and Rocket Pharma expand LVV manufacturing partnership
The expanded agreement will ensure Rocket Pharma's supply of lentiviral vectors across its pipeline -
News EU secures additional 100 million doses of Pfizer-BioNTech vaccine for 2021 delivery
Extra supply will bring total number of doses scheduled to be shipped to EU this year to 600 million -
News Sartorius expands manufacturing capacities in China and the UK
The expansions will enable the company's local customers to test equipment directly at the manufacturing site -
News CPHI Japan open for business!
Informa Markets' first in-person trade show of the year welcomes attendees in Tokyo as face-to-face interaction recommences among pharma community -
News Lonza and Junshi Biosciences expand biologics manufacturing collaboration
The antibody-based products will be expressed using Lonza's GS Xceed Gene Expression System
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance