Innovating in the viscosity design space with a new 2.25 mL autoinjector platform
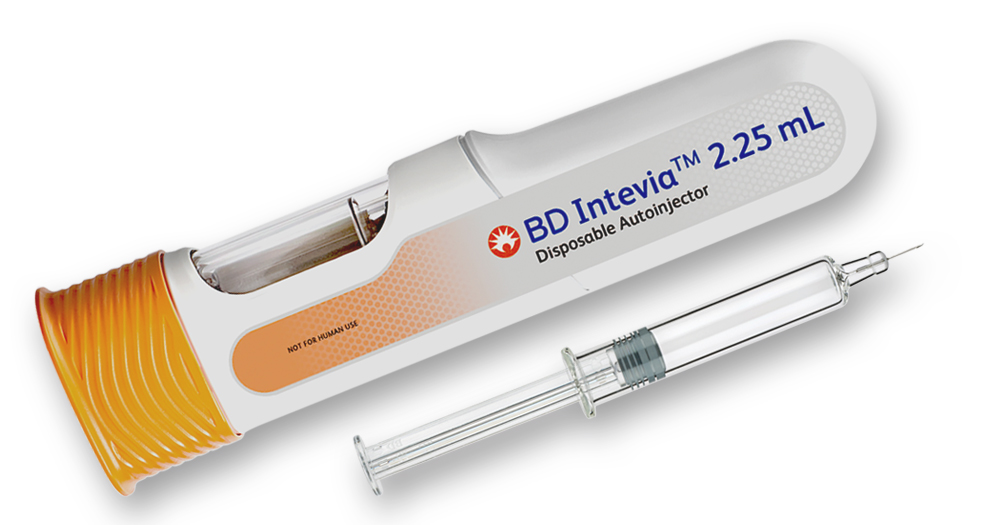
Extending its patient-centric BD Intevia™ Disposable Autoinjector platform, BD builds on past successes while meeting the requirements of new, highly viscous, high-volume biologics for chronic diseases
Multi-disciplinary teams at BD set out to develop an autoinjector platform ready to meet the requirements of the highly viscous, high-volume (1) biologics that are upcoming in the chronic disease space. The result is an autoinjector platform that delivers an enhanced experience for patients (2), extends the design space beyond traditional subcutaneous (SC) delivery for biologics and can lower development risks for biopharmaceutical partners.
Large molecules
The market for monoclonal antibody-based drugs (mAbs) has undergone significant growth. These drugs are often characterised by large molecules requiring administration in large injection volumes and/or high concentrations. These proteins require parenteral administration via intravenous or SC injection, due to their low bioavailability via other routes. This can present a challenge for existing SC autoinjector technologies that have been limited to lower viscosities and volumes.
With the launch of the BD Intevia™ disposable autoinjector platform, BD offers a range of devices combining an autoinjector and a prefillable syringe in one integrated system specifically designed for high-viscosity, high-volume biologics. Created using BD’s quality-by-design approach, the BD Intevia™ 2.25 mL disposable autoinjector is a robust and integrated two-step, push-on-skin device that serves a broad design space, providing pharmaceutical companies with additional degrees of freedom and flexibility in drug formulation. The BD Intevia™ 2.25 mL disposable autoinjector delivers drugs with viscosities up to 40 cP safely and effectively. (3, 4) Pharmaceutical companies can adapt the BD Intevia™ 2.25 mL disposable autoinjector to deliver a range of drug volumes and viscosities without any need for customisation of the system or of its components. (3)
8 mm needle technology
The BD Intevia™ 2.25 mL disposable autoinjector integrates the BD Neopak™ XtraFlow™ 2.25 mL glass prefillable syringe, featuring innovative 8 mm needles with Thin Wall (TW) cannula technology (Figure 1). BD Neopak™ XtraFlow™ glass prefillable syringes are characterised by breakage resistance, design quality, autoinjector compatibility and dimensional precision for dose accuracy. (3) By directly addressing many of the risk factors that can cause development delays, (5, 6) BD Neopak™ XtraFlow™ glass prefillable syringes contribute to reduced time to market and optimised commercialisation of biopharmaceuticals. (6)
The BD Neopak™ XtraFlow™ glass prefillable syringe simplifies the burden of device integration faced by pharmaceutical companies. With its robust integration into the BD Intevia™ disposable autoinjector platform, the BD Neopak™ XtraFlow™ glass prefillable syringe helps eliminate the risks associated with the integration of components from diverse manufacturers, (4) as well as the risks associated with the customisation of the secondary device.
The integration of the BD Neopak™ XtraFlow™ glass prefillable syringe supports simpler adaptation to drug volumes and viscosities and reliable combination product performance. This may contribute to cost savings for biopharmaceutical partners, (6) while also contributing to comfort, safety and convenience for patients. (1, 4)
Customization not required
With BD Intevia™ disposable autoinjectors, one device configuration addresses a broad range of viscosities and volumes without the need to customise system and subsystem components. This high degree of flexibility is made possible by accommodating the demands of different viscosities and volumes solely through the parameters of the needle integrated into the Neopak™ XtraFlow™ glass prefillable syringe. By using a shorter needle and increasing its inner diameter without increasing the external diameter (by reducing the thickness of the needle wall), injection force is reduced by up to 46% compared with a commonly used 12.73 mm needle. (7) This is achieved without compromising needle robustness (8) or increasing pain perception. (9)
To manage a range of viscosities and injection volumes, BD Neopak™ XtraFlow™ 2.25 mL glass prefillable syringes are available with 27G Ultra-Thin Wall, 27G Special-Thin Wall and 29G Thin Wall 8 mm needles. Thanks to BD Intevia™ 2.25 mL disposable autoinjector and BD Neopak™ XtraFlow™ 2.25 mL glass prefillable syringe system, a single autoinjector is equipped to handle biologic formulations with viscosities ranging from aqueous to 40 cP and fill volumes up to 2.25 mL.
Patient and partner centricity
The BD Intevia™ disposable autoinjector platform supports pharmaceutical companies that wish to take the lead in patient comfort (1) in the injection of biologics at dose volumes of up to 2 mL via SC delivery. The platform is the direct of result BD’s patient-centric design approach and commitment to developing drug delivery solutions tailored to home care, with the aim to improve patient adherence to chronic disease treatments.
BD offers a range of services and data to support its customers’ combination product development programs. BD also performs extensive preclinical and clinical studies to help de-risk combination product development processes and enable a reduced time to market.
Globally recognized for design excellence
The BD Intevia™ autoinjector platform has earned BD the Frost & Sullivan Global 2020 Technology Innovation Award in the autoinjector drug delivery device market. It is also the recipient of the global Good Design Award, the world’s most prestigious, well-recognised and oldest design awards programme, organised annually by The Chicago Athenaeum Museum of Architecture and Design in cooperation with the European Centre for Architecture, Art, Design and Urban Studies and Metropolitan Arts Press, Ltd.
REFERENCES
1. Li Z, Easton R, “Practical Considerations in Clinical Strategy to Support the Development of
Injectable Drug-Device Combination Products for Biologics”, MAbs, 2018, Vol 10(1), pp 18–33.
2. “Validation Study of BD Intevia™ 1 mL Two-Step Disposable Autoinjector”. Internal Study, 2019; Becton, Dickinson and Company.
3. “Design Input Specification for BD Intevia™ 2.25 mL”. Internal Report, 2017; Becton, Dickinson and Company.
4. “PHS-20-LVA06 HF Late Formative Study for BD Intevia™ 2.25 mL Two-Step Disposable Autoinjector formative study”. Internal Study, 2020; Becton, Dickinson and Company.
5. Rathore, N, et al, “Variability in Syringe Components and its Impact on Functionality of Delivery Systems”. PDA J Pharm Sci Technol, 2011, Vol 65(5), pp 468–480.
6. “System Integration Challenges in Combination Products for Injectable Drugs”. Internal Study, 2019; Becton, Dickinson and Company.
7. “Injection Time and Ejection Force Calculation”. Internal Study, 2020; Becton, Dickinson and Company.
8. “BD Neopak™ XtraFlow™ Needle Resistance Simulation”. Internal Study, 2020; Becton, Dickinson and Company.
9. Pager, A, et al, “User Experience for Manual Injection of 2 mL Viscous Solutions is Enhanced by a New Prefillable Syringe with a Stacked 8 mm Ultra-Thin Wall Needle”. Expert Opin Drug Deliv, 2020,
Vol 17(10), pp 1485–1498.

Related News
-
News Patients vs Pharma – who will the Inflation Reduction Act affect the most?
The Inflation Reduction Act brought in by the Biden administration in 2022 aims to give better and more equitable access to healthcare in the USA. However, pharma companies are now concerned about the other potential costs of such legislation. -
News CPHI Podcast Series: What does the changing US Pharma market mean for industry and patients alike?
In this week's episode of the CPHI Podcast Series Lucy Chard, Digital Editor for CPHI Online is joined by James Manser to discuss the political and market changes in the US pharma field. -
News CPHI Barcelona Annual Report illuminates industry trends for 2024
The CPHI Annual Survey comes into it’s 7th year to report on the predicted trends for 2024. Over 250 pharma executives were asked 35 questions, with their answers informing the industry landscape for the next year, spanning all major pharma marke... -
News Which 10 drugs are open to price negotiation with Medicare in the USA?
The Centres for Medicare & Medicaid Services, under the Biden administration in the USA, has released a list of the 10 drugs that will be open to price negotiations as part of the new legislation under the Inflation Reduction Act (IRA). -
News EU Medical Devices Regulation causes unintended disappearances of medical devices for children, doctors state
Doctor groups and associations have appealed to the EU to correct the EU Medical Devices Regulation law that may cause unintended shortages of essential drug and medical devices for children and rare disease patients. -
News 10 Major Drug Approvals So Far in 2023
Last year, 37 novel drugs were approved by the FDA, this was a high number for such a category, and covered many fields including oncology, demonstrating how promising further research is, and how it is only continuing to build. To date, there are alre... -
News Detecting Alzheimer's disease with a simple lateral flow test
A novel rapid diagnostic test for early-stage Alzheimer's disease has been developed using a biomarker binder from Aptamer Group along with technology from Neuro-Bio, the neurodegenerative disease experts. -
News CPHI Podcast Series: outsourcing and manufacturing trends
Listen to the CPHI Podcast Series this June to hear Gil Roth of the PBOA speak with Digital Editor Lucy Chard about the biggest trends and topics to watch in pharma outsourcing and manufacturing at the minute.
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance







