Market News
Market news
-
News Spanish CDMO invests €200k to protect clients' products
Investment will enable Idifarma to both serialise commercial batches manufactured by them for clients and offer standalone serialisation services. -
News Metrion Biosciences awarded £200k funding to further develop in-house drug discovery pipeline
Funding will support further development of recently acquired small molecule assets for treatment of autoimmune disease. -
News Lab automation systems for faster scientific breakthroughs
PAA showcases its complete range of automation solutions, suitable for any type of laboratory or application, at SLAS2018. -
News Sartorius scoops double award at Fisher Scientific European Sales Conference
Company also promoted to Platinum supplier status in Europe. -
News A powerful new tool for the separation of charged species
A new analytical tool, Electrical Asymmetrical Flow Field-Flow Fractionation (EAF4) is showing much promise in biopharmaceutical and nanoparticle applications. -
News New Pall business unit launches to focus on end-to-end drug manufacturing models
Pall Biotech to focus on enabling drug manufacturers to rapidly transition from pre-clinical to commercial manufacturing. -
News Plastic: pharma's friend or foe?
Love it or loathe it, plastic is here to stay, but that doesn't let the pharmaceutical industry off the hook. -
News Cambrex expands process R&D capabilities across North America
The expansion at Charles City is in parallel to the investments announced in 2017 at Cambrex’s facility in High Point, North Carolina. -
News Ablynx - Sanofi's new acquisition
Strengthens Sanofi's R&D strategy with innovative Nanobody technology platform. -
News Reig Jofre launches sleep supplement in France
Forté Pharma's new nutritional supplement combines natural extracts: valerian, passionflower, and vitamins B3 and B6, which contribute to prolong sleep for 8 hours. -
News Companies warned for selling unapproved opioid cessation products using deceptive claims
The companies have 15 days to respond to the FDA and FTC. -
News Collaboration expanded to explore synthetically feasible chemical space
Enamine REAL database of synthetic compounds accessible via UCSF’s online ZINC platform. -
News Shire receives FDA approval for tech transfer of Cinryze
Commercial manufacturing of Cinryze drug product in Vienna to begin in the first quarter of 2018. -
News Partnership in AI and machine learning for target discovery
LifeArc and Milner Therapeutics Institute to develop, validate and integrate cutting-edge machine learning approaches into drug discovery processes. -
News Sanofi acquisition expands its leadership in rare diseases
Sanofi to acquire Bioverativ for $11.6 billion. -
News Sandoz and Biocon to collaborate on next-generation biosimilars
Collaboration will leverage combined strengths of development, manufacturing and commercialization of biosimilars. -
News Charles River acquisition enhances discovery expertise
KWS increases Charles River's ability to support clients’ early-stage drug research in critical therapeutic areas. -
News Taking the complexity out of global track and trace requirements
With time running out, Meditraq launches to simplify serialisation data challenge. -
News Croda acquires marine biotechnology company
Through the acquisition of Nautilus Biosciences, Croda will utilise the company's innovative science for applications across all its market sectors. -
News Cambrex opens large-scale capacity expansion at its Karlskoga facility
Opening ceremony marks long-term supply agreement between Cambrex and AstraZeneca for a key intermediate. -
News Japanese pharma – no longer ‘lost in translation’
New Year CPHI Japan report predicts 2018 will be a transformative year for Japanese pharma, with early movers likely to benefit. -
News 2017: A year of innovation and advances
A summary and highlights of new drug therapy approvals and other drug therapy advances in 2017 by FDA's CDER. -
News New active ingredients provide to correct the signs of skin and hair ageing
Three anti-ageing and anti-stress active ingredients for manufacturers of skin care product -
News New automated VPT systems for topical drug performance testing
The MedStat-HT and MedFlux-HT help to de-risk development programs and shorten development timelines. -
News BYDUREON BCise Injectable medicine now available in the US for type-2 diabetes patients
The new formulation is proven to reduce blood sugar levels and may have the added benefit of weight loss. -
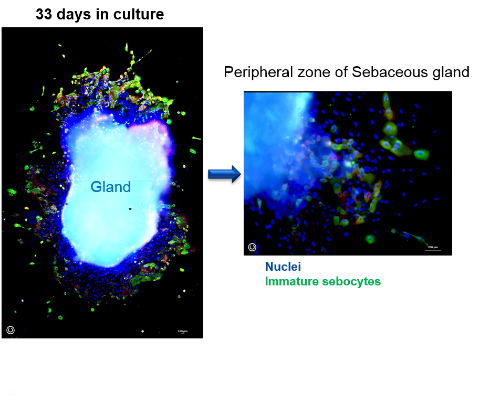
News 3D human sebaceous glands technology for skin care applications
New 3D technology provides a powerful platform for skin care researchers wishing to study the function of sebaceous glands, in relation to a range of age-related, microbial and inflammatory skin disorders. -
News Revolutionary medical innovations being unleashed in Canada MaRS discovery district
Health investors are paying attention to Canada, particularly to Toronto’s hot market. -
News Takeda to acquire TiGenix
Acquisition will expand Takeda’s late-stage pipeline and leadership in gastroenterology. -
News Sangamo and Pfizer collaborate to develop zinc finger protein gene therapy for ALS
"The precision and flexibility of zinc finger proteins enables targeting of virtually any genetic mutation." -
News SSB to equip Abzena's US-based development and manufacturing sites
SSB will provide both facilities with end-to-end process solutions in single-use format. -
News Momenta and Mylan to development proposed biosimilar to Eylea
Targeting the initiation of a pivotal patient clinical trial in the first half of 2018. -
News Junior Filters for space restricted applications
Ideal for a wide variety of pharmaceutical applications. -
News Scholar Rock announces $47 million Series C financing to advance innovative pipeline
SRK-015 to move into clinical development for Spinal Muscular Atrophy (SMA) in first half of 2018; development in additional neuromuscular disorders to follow. -
News Recipharm acquires remaining shares in Nitin Lifesciences
The transaction will allow Recipharm to combine and better exploit operational synergies with the company's other Indian business. -
News Juniper Pharma Services’ co-founder awarded CBE in the Queen’s New Year’s Honours List
Martyn Davies recognised for his contribution to UK science and his ground-breaking achievements in pharmaceutical research and drug development. -
News Novel Sorrento CAR-T technology a potential “game-changer
Company envisions the development and IND filings of multiple “next-frontier” CAR-T programs in 2018 and beyond, which will position the company as a potential major player in the fast-growing CAR-T space of immunotherapies against cancers. -
News ANVISA approves Biocon and Mylan's biosimilar Trastuzumab
Libbs will commercialize the product in Brazil under the brand name Zedora. -
News FDA approves first generic for Estrace Cream
Mylan now offers estradiol in four delivery methods. -
News Shire files for FDA approval of a new plasma manufacturing facility
The additional capacity from this site is a key element to support the growth in the company's Immunology franchise. -
News Teva launches generic version of Reyataz in the US
The exclusive launch of Teva's generic version of Reyataz marks the company's fifth generic product offering for the treatment of HIV-1 infection. -
News Novartis drug Tasigna approved by FDA
First and only CML therapy with Treatment-free Remission data in its label. -
News FDA approves the only OTC eye drop with low-dose Brimonidine to treat eye redness
Clinical studies showed 95% symptom improvement at one minute and reduced redness for up t 8 hours. -
News AMSBIO introduces highly efficient substrate for culturing ES/iPS cells
iMatrix recombinant Laminin-511 E8 protein fragments permit ES/iPS cells to be maintained in xeno-free, feeder-free culture conditions enabling easy application in medical trials. -
News Allergan acknowledges Appeals Court decision for Combigan
Allergan plans to file petitions for rehearing of the appeals court decision and has asserted a new Orange Book patent against Sandoz's generic version of Combigan. -
News Crown Bioscience and JSR Corporation to merge
Merger integrates Crown Bioscience’s leading translational technology platform providing drug target validation, efficacy testing and patient response characterization with JSR’s IVD solutions, GMP manufacturing capabilities and worldwide distribution ... -
News Biogen and Ionis collaborate to identify novel therapies for the treatment of SMA
New collaboration agreement to identify new antisense oligonucleotide drug candidates. -
News FDA clears myPKFiT for ADVATE to help personalize care for hemophilia A
First and only FDA-cleared PK dosing software to support healthcare professionals in creating personalized dosing regimens for patients 16 and older with hemophilia A. -
News Major changes for bio supply forecast in 2018 by bioLIVE
Integration of supply chain a critical issue as industry globalises, with a resurgence in European manufacturing and biotech markets forecast. -
News Hikma signs licensing agreement with Celltrion for third biosimilar product in MENA region
Truxima is mAb biosimilar to Roche's MabThera (rituximab). -
News Teva to axe 25% of its total workforce
Total cost base to be reduced by $3 billion by the end of 2019. -
News Femarelle launches new women’s supplement line in US
Targeted formulas support symptoms before, during and after menopause. -
News Lilly and Rimidi to make diabetes management easier
Companies will strive to make diabetes management easier through effective use of connected devices and a diabetes management software platform. -
News Major breakthrough for new haemophilia A treatment
Drug trials provide mind-blowing results. -
News New formulation of Oncaspar receives Marketing Authorization in Europe for patients with ALL
Freeze-dried, or lyophilized, formulation aims to improve supply management by providing the same dosing regimen as liquid Oncaspar but with a three-times longer shelf life of up to 24 months. -
News CMC Biologics and Harpoon Therapeutics collaborate on exciting new TriTAC molecules
First clinical candidate, HPN424, is in development for the treatment of metastatic prostate cancer and expected to enter Phase I clinical trials in 2018. -
News SGS introduces GMP DNA sequencing service at its Glasgow, UK, laboratory
Service complements existing services for this testing, to provide comprehensive analysis and characterization solutions for clients involved in the production and manufacturing of biopharmaceutical products. -
News Making better business decisions and optimizing process control
New version of SIMCA -advanced data analytics and visualization program - unlocks the hidden gems that hold the secret to better decision-making and greater business success. -
News SGS launches new large molecule bioanalytical services
Investment builds on company's expertise in small molecule analysis and the use of LC/MS-MS techniques. -
News Expansion of CSL Biotech facility drives advanced manufacturing growth
The development will help meet growing global demand for albumin. -
News Global ADC Bio clients to benefit from new dedicated bioconjugation facility
The design, planning and building work commences immediately and will deliver a purpose-built, dual-stream facility. -
News M&A activity and investor cash expected to re-invigorate pharma and biotech in 2018
Public and private investors look set to remain supportive of innovative, early-stage drug developers. -
News New TPK 2100 Critical Flow Controller for dry powder inhaler testing
A highly automated system to speed up testing, improve reproducibility, and enhance data output and recording. -
News API and drug product CDMO reports rapid growth in MAH programs in China
Since the MAH pilot launch, the company has undertaken eight development programs. -
News Bluebird Bio's acquires manufacturing facility to produce lentiviral vectors
Company executing long-term strategy to develop broad manufacturing capabilities for both vector supply and drug product supply to support clinical development and commercialization across pipeline. -
News Major pharma company to make significant investment in the UK
MSD's announcement to establish UK Discovery Centre in London an 'endorsement' of the government's Industry Strategy. -
News Swedish CDMO recognised for its commitment to its French operations
Recipharm's four French facilities now serve over 50 of its customers worldwide, including a number of top 20 pharma firms. -
News Recipharm equips a further three facilities for US serialisation
CDMO's serialisation capabilities in place ahead of US deadline to avoid potential problems for our customers. -
News New clarification platform delivers robust, accelerated performance across multiple mAb processes
The Stax mAx, a single-use harvesting platform, minimizes the impact of process variability between batches. -
News Statistical monitoring services in response to ICH GCP E6(R2) addendum reduce time and resource
Offers customers a comprehensive, risk-based approach to the monitoring of data quality throughout the course of a study. -
News Innovative reusable autoinjector provides additional administration option for Enbrel users
Amgen launches the Enbrel Mini single-dose prefilled cartridge with AutoTouch reusable autoinjector, which is ergonomically designed for patients. -
News Cardinal Health sells its China business to Shanghai Pharma
The acquisition will facilitate the growth of Shanghai Pharma's pharmaceutical manufacturing business. -
News Leveraging continuous manufacturing technologies based on current resources and future goals
Unique training courses designed around real-life situations, with hands-on access to enabling technologies. -
News Pharma set for prosperous 2018 as 44,500 attend CPHI Worldwide
Germany tops quality-ranking categories in the inaugural CPHI Global Pharma Index. -
News Company's new identity integrates services typically found in disparate CDMOs and CROs
Quotient Sciences Translational Pharmaceutics platform integrates formulation development, real-time adaptive GMP manufacturing and clinical research. -
News India ranked by international pharma as the third most competitive nation
Perception of India’s API manufacturing seems to be improving. -
News Poor aggregation will see ADC targets fail or face long delays
Experts at ADC Bio warn of impending problems in the ADC pipeline with millions wasted in development costs. -
News Copley Scientific introduces new, upgraded breath simulators for inhaled product testing
The upgraded BRS simulators make it even easier to apply specified inhalation profiles during OIP testing. -
News Key trends in formulation development for 2018
Controlled-release technologies will continue to be focal point, whereas techniques to improve solubility are likely to gain popularity. -
News Vetter expands its footprint in Asia Pacific 'sweet spot'
The pharmaceutical service provider will be better positioned to support local and global customers, helping them to meet stringent development, manufacturing and packaging requirements of their injectable drugs. -
News Recipharm to end operations in two facilities in Sweden
Company's decision affects approximately 225 staff, specialised in tablet manufacturing, and in sachet and stick pack filling, primarily for powders and granules. -
News Cambrex boosts small-scale capacity to increase flexibility
Two 500-gallon glass-lined reactors installed to reduce potential bottlenecks. -
News Automating the process of screening excipients for solubilising drug candidates
The process, which uses significantly less materials than the manual alternatives, will ultimately improve drug developers’ clinical pathway. -
News NousCom raises €42 million to develop off-the-shelf cancer vaccine
Financing to advance its pipeline of neoantigen cancer-based immunotherapies to the clinic. -
News Venezuela’s emerging pharmaceutical markets threatened
Economic and political turmoil along with inefficient patent laws and drug pricing policies are major barriers. -
News Celonic acquires Glycotope’s biomanufacturing facility
Combined technology assets will provides pharmaceutical and biotech customers the unique opportunity to select a tailored expression system. -
News Mylan response to announcement of proposed amendments to civil complaint
Company says it has found "no evidence of price fixing on the part of Mylan or its employees". -
News Novartis announces planned acquisition of Advanced Accelerator Applications
Acquisition would add Lutathera, a first-in-class RadioLigand Therapy approved in Europe and under review in the US for neuroendocrine tumours. -
News Normon Laboratories selects TraceLink to comply with EU FMD
TraceLink selected for its demonstrated EU and country compliance capabilities and enterprise scalability. -
News Reig Jofre's nutritional supplements line expands its product portfolio
Forté Pharma expands its energy and health segments with the launch of eight references in the French market, as a result of its product development strategy. -
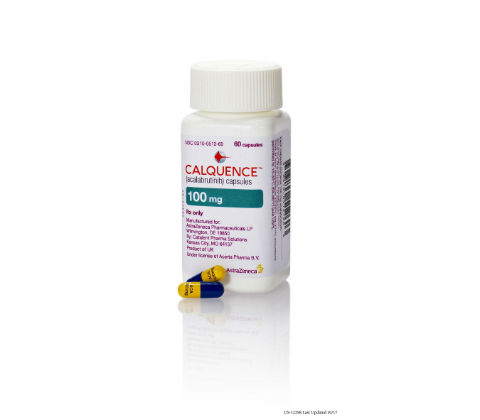
News FDA approves AstraZeneca's Calquence for adult patients with mantle cell lymphoma
Accelerated approval of Bruton tyrosine kinase inhibitor in MCL marks AstraZeneca's entry into the treatment of blood cancers. -
News ChargePoint Technology certified for supplying solutions for hazardous environments
Company now boasts having the highest quality standards from both its in-house manufacturing and its external partners, who are also audited and certified. -
News Eurofins creates one of the largest dedicated testing sites of its kind in the UK
£4 million investment will allow the Biopharmaceutical Product Testing business to expand its finished product and raw materials testing and increase capacity to deal with higher volumes. -
News CDMO extends spray drying capacity for aqueous and organic solvent-based formulations
Micro-Sphere adds GEA PSD-3 spray dryer in response to increased demand in spray drying. -
News Spinraza scoops Best Biotechnology Product award
Biogen and Ionis receive 2017 Prix Galien USA Award for extraordinary achievement in scientific innovation. -
News New dedicated biopharma event launches alongside CPHI Worldwide 2018 in Madrid
New event to create unique synergies between the parallel worlds of large, small molecules and contract services. -
News Mylan wins UK court ruling related to Copaxone 40 mg/mL patent
Court finds all claims of Teva's patent relating to Copaxone invalid based on obviousness. -
News SSB wins Frost & Sullivan Award for Customer Service Leadership in Bioanalytical Contract Testing
Company recognised for its innovative approach to assay development, which provides customers with a portfolio of off-the-shelf assays, saving them time and money. -
News CPHI Worldwide announces the winners for its 14th Pharma awards
19 winners chosen from 200 entries, received from nearly 30 countries worldwide. -
News Report forewarns of intense battle between innovators and biogeneric companies for the next 3 years
Experts state pharmaceutical regulation will shift due to increased data dependence, warn regulatory oversight is slowing six sigma adoption, and forecast increased generic options 2025 onwards. -
News India’s generics exports expanding 22% per year
Country accounts for over 20% of global production with $20 billion exports forecast by 2020. -
News Recipharm equips Lisbon facility for US and European serialisation
The Lisbon facility brings the CDMO to over a third of the way through its implementation project. -
News Cambrex adds large-scale manufacturing capacity at its Charles City, Iowa Facility
Additional capacity enables the company to take on new customer projects, as well as offering the flexibility for projects to be transferred in from other Cambrex sites. -
News Lilly to invest $72 million in diabetes manufacturing in Indianapolis
Investment is part of the company's anticipated $850 million in US capital projects in 2017. -
News Vetter to expand its secondary packaging service capacity to meet rising demands
Investment will contribute to a one-stop solution approach for customers. -
News I Holland to share its expertise at Compression Tooling Seminar
Comprehensive 2-day event takes attendees through the most common problems found in modern tablet production, from sticking and picking to weight control and capping. -
News Seebri Neohaler launches in the US
Company eligible to receive royalties from Novartis on net sales of the product. -
News Will Novo Nordisk's semaglutide be its next blockbuster?
Semaglutide receives positive 16-0 vote in favour of approval from FDA Advisory Committee. -
News Current drugs pipeline is discouraging and slowing simple manufacturing innovations
Experts predict PAT/QbD and CM adoption may take 10 years, with smaller generic players to drop out of the market if unable to innovate in time. -
News TraceLink hits industry milestone with 100 CMOs serialization-ready on the Life Sciences Cloud network
TraceLink, Sharp and Major Pharma Brand to discuss trade partner connectivity at CPHI Worldwide. -
News New European Council of regulatory professionals set to shape and support the profession
New European Council will support RAPS’ growing member base and volunteer network across Europe to ensure compliance with global regulations and to drive regulatory excellence -
News STA Pharmaceutical to open new transition metal catalysis center in Changzhou, China
The center will allow STA to introduce transition metal catalyst-screening technology to their existing small-molecule process development and manufacturing platform. -
News SIRION Biotech and Vibalogics partner to offer complete AAV services
The partnership will be able to cater to virtually all customer needs for viral vector services, from initial vector engineering and development to GMP production. -
News Mylan invalidates Allergan's patents on Restasis
US District Court for the Eastern District of Texas decides all asserted claims of the patents relating to Restasis invalid based on obviousness. -
News Amneal and Impax to combine
Combination creates diversified pharmaceutical company with 5th largest generics business in the US. -
News Batavia expands its viral vector and cleanroom facilities to stay on top of demand
Investments will help the company to bring candidate biopharmaceuticals from bench to clinic. -
News CPHI Worldwide report highlights unpredictability in CDMO growth over next 5 years
Future acquisitions will not be dominated by big deals and large mergers, but rather smaller commentary services and niche technologies to fill specific technology asset gaps. -
News SGS and Bavarian Nordic to develop a novel and differentiated challenge model for RSV
Project will build upon the results of Phase II trials undertaken by Bavarian Nordic. -
News Maximising API performance with high purity excipients
Croda on hand at CPHI to increase API delivery and improve API stability. -
News Cambrex invests in generic API development and manufacturing capabilities at its Milan site
Investments include the installation of development and analytical equipment to support the development of highly potent APIs. -
News New filling and closing machine AFG 5000 from Bosch
Compact and high-speed powder filling- Output of up to 480 vials per minute
- Number of filling points can be individually selected
- Optimal use of work stations thanks to the new transport system
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance








.jpg)


.jpg)


.jpg)

.png)


.png)
.png)
.png)


.png)


.png)



.jpg)

.png)
















.png)



.png)




.jpg)

.jpg)




.jpg)
.png)
.png)

.png)

.jpg)









.png)

.png)
.png)



.png)



.png)




.png)

