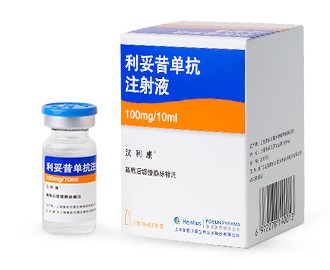
汉利康® (pronounced as Han-Li-Kang) Rituximab Injection. It is the first-ever China-manufactured biosimilar approved by the NMPA for the treatment of non-Hodgkin lymphoma (NHL). Meanwhile, we are also conducting a Phase 3 clinical trial for rheumatoid arthritis (RA) for HLX01 in China. 汉利康® (rituximab injection) was granted New Drug Application (NDA) approval by the NMPA in February, 2019, which also makes it the first biosimilar drug approved in China in accordance with the Biosimilar Guidelines.Other Research Progress:
2 Products under China NDA/EU MAA Review
14+6 Products/Combo Therapies under R&D
20+ Global Clinical Studies