22
Jan
2025
Vetter Pharma-Fertigung GmbH & Co. KG
Exhibitor at Pharmapack Europe 2025 stand H37
About Us
Categories

-
DE
-
2015On CPHI since
-
3Certificates
-
5000+Employees

Company types
Primary activities
Event information
Pharmapack Europe 2025
-
22 Jan 2025 - 23 Jan 2025
-
Paris Expo, Porte de Versailles - Hall 7.2 | Paris, France
-
Visit us at stand H37
Products Featured at Pharmapack Europe 2025
-
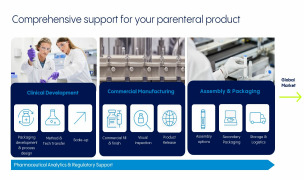
Product Drug Product Development
Our Vetter Development Services help biopharma customers lay a foundation of scalability, quality, and sustainable value for drug product profiles. We support drug developers in navigating key steps, decisions, and transitions in the product development cycle. This includes primary packaging evaluation, pr... -
Product Aseptic filling and visual inspection
For over 40 years, our fill and finish services have helped to keep the world supplied with injectable therapies, many of which are lifesaving. Our partnerships with customers start in the first steps of clinical manufacturing through commercial-scale filling and beyond, including management throughout the... -
Product Device Assemply and Packaging
The injectable drug market is rapidly evolving and includes an array of delivery devices from autoinjectors to pens and other intuitive formats. We provide partnership for drug owners driven by the top-quality, complex considerations and flexibility expected. These services include assembly process des... -
Product Analytical Services
From a drug product’s first raw materials to its release-ready batches, our experts rigorously evaluate and verify a product’s quality at key steps throughout the manufacturing process. Analytical services include material testing to verify product quality from the early stages of development, cGMP-com... -
Product Regulatory Support
Our Chemistry, Manufacturing and Controls (CMC) specialists help customers manage the product’s compliance at every step, from clinical development to market authorization. With decades of experience, our experts provide support in navigating regulatory milestones, including site registration, product ... -
Product Logistic Service
We have established a proven and optimized process along the logistic chain and are dedicated to provide services from API storage to a ready-to-dispatch product. Our long and successful history has enabled us as experts in storing, packaging and worldwide cold chain shipment for injectables. Our compr... -
Product Corporate Service Portfolio
We place heightened focus on your success as your strategic partner.
We produce aseptically prefilled syringes, cartridges, vials and dual-chamber systems as a globally operating CDMO partner. We are a family-owned, independent company rooted in 70+ years of history. We do not manufactu...
Vetter Pharma-Fertigung GmbH & Co. KG Resources (9)
-
Video About us I Vetter Corporate Image Video
Our values make us as pharmaceutical service provider, employer, and family business unique. Find out what stands behind the Vetter name. -
Video Vetter Development Service Chicago | US-Based Clinical Manufacturing Site
Our US-based clinical development site offers comprehensive services and know-how for a wide variety of compounds, including process development, cGMP clinical batches, regulatory support and more. This state-of-the-art facility features 50K square feet of specialized lab space, 2 clean rooms, and the expertise of over 100 clinical development professionals. -
Video Discover Vetter Secondary Packaging & Device Assembly
We can package a wide variety of drug delivery systems and formats and offer several services such as cartoning (all paper), blistering, labeling, serialization and aggregation , testing. Our dedicated engineering department specializes in developing novel packaging processes for safety device and delivery systems like pens and autoinjectors. -
Whitepaper e-book I Going to Market in an Autoinjector
How to prepare for the device assembly & packaging process -
Whitepaper e-book I Checklist Clinical Manufacturing
Get your product to the clinic with confidence
The shift from preclinical to clinical development is a critical step in the development of your drug product.
During this transition, you not only have to significantly upscale production of your product, but also make many strategic decisions that will lay important groundwork for your regulatory submission, commercial production of your product, and many other key components of your products life-cycle.
This eBook will help you build a plan that not only streamlines your products path to the clinic but also sets you up for long-term development and market success - starting with a proven 6-step process for filling your critical clinical batch.
-
Brochure Unlock the full potential of your injectable product
For over four decades, pharma and biotech companies around the world have relied on us to put their parenteral medications on a path to success. We’re proud to help our customers advance their valuable products with innovative services, cutting-edge technology, and flexible, robust, scalable processes that set our partnership apart. -
Podcast Recap and Forecast 22/23
Rizwan Chaudhrey chats with experts across the world of Life Science, Pharma and Bio-Pharma on all things related to drug discovery, development, manufacturing, leadership, entrepreneurship and other topics. Now he launched a new series called reflections and forecast 2022/ 2023.
In his first episode he chats with Carsten Press, SVP Key Account Management, Supply Chain Management and Marketing, about Vetter Pharma, its key achievements and challenges in 2022 as well as about how the CDMO changed since the pandemic. Furthermore, they talk about predictions for the Life Science/Pharma sector in 2023, market trends and developments and what can be expected from Vetter Pharma in 2023 -
Podcast From a Pharmacy to a 1bn family-owned CDMO
Peter Soelkner, Managing Director at Vetter, sat down with Raman Sehgal of the “Molecule to Market” podcast to share his personal background, the history of Vetter, and the ambitious goals the company is striving towards today. -
Webinar CMDO Insights Into Launching an Autoinjector
The rising demand for at-home self-administered therapies has made patient-friendly formats, such as autoinjectors, a necessity for drug manufacturers. However, the intricacies of manufacturing these devices pose significant challenges. This presentation offers valuable insights into planning the device assembly and packaging process, enabling drug owners to navigate this evolving landscape with confidence and efficiency.
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance














-file108371.jpg)

-file142209.jpg)
-file145594.png)