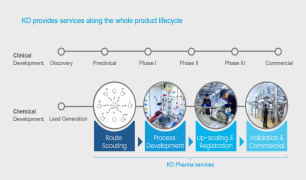
7
May
2024

KD Pharma Group
Exhibitor at CPHI North America 2024 stand 800
About Us
Categories

-
CH
-
2021On CPHI since
-
250 - 499Employees

Company types
Primary activities
Event information
CPHI North America 2024
-
07 May 2024 - 09 May 2024
-
Pennsylvania Convention Center, Philadelphia
-
Visit us at stand 800
Products Featured at CPHI North America 2024
-
Product CONTRACT MANUFACTURING - SMALL MOLECULES
KD Pharma’s professional expertise, state-of-the-art assets and proven track record in regulatory compliance allow us to become your preferred CDMO solutions partner.
Your Agile Experts
• Responsive to our customers’ needs in a more dynamic customer-centric way • Adaptive to the most diverse re... -
Product ASYMMETRIC HYDROGENATION
We extensively address the specific needs of the pharmaceutical, food, cosmetics, agrochemicals and specialty chemicals industries with our capabilities in Transition Metal Catalysis (TMC) as applied to asymmetric hydrogenation. Complex active pharmaceutical ingredients (API’s) are often stereochemica... -
Product API & INTERMEDIATES
The CDMO Division of KD Pharma excels in the production of drug intermediates and API drug substances. We offer our customers the full spectrum of drug services: • R&D development material for preclinical studies • Pilot plant for phase I, II and III supplies • Large scale p... -
Product HIGH-PRESSURE HYDROGENATION
Sophisticated reduction technologies:The CDMO Division of KD Pharma operates highly corrosion resistant Inconel® facilities to perform hydrogenation under pressure up to 64 bar (928 psig) and temperatures up to 150°C from lab to industrial scale (4 m3). Both homogeneous as well as heterogene... -
Product SIMULATED MOVING BED CHROMATOGRAPHY (SMB)
KD Pharma is a contract manufacturer that develops products in the pharmaceutical space by employing state-of-the-art technology. Since its inception in 1988, KD Pharma has focused on utilizing cutting edge technologies to develop and manufacture exceptional quality APIs. These twin pillars of technology a... -
Product CARBONYLATION / C-C & C-N COUPLING
We extensively address the specific needs of the pharmaceutical, food, cosmetics, agrochemicals and specialty chemicals industries with our capabilities in Transition Metal Catalysis (TMC). Complex active pharmaceutical ingredients (API’s) are often stereochemically demanding: use of modern synthetic metho... -
Product CYANATION OF ANILINES AND HALOAROMATICS
KD Pharma has been performing a number of different cyanation reactions on industrial scale. Among those, cost efficient and selective cyanation of amino-aromatics through diazotization and subsequent Sandmeyer reaction is standard practice.In addition, Copper(II)-promoted direct cyanation of haloaromatics... -
Product DISTILLATION
Modern distillation technologies:The CDMO Division of KD Pharma operates different multifunctional distillation facilities from pilot scale to industrial scale volumes. In combination with KD Pharma’s outstanding and highly flexible manufacturing capabilities, we can produce various high purity liquid prod... -
Product HAZARDOUS AND MALODOROUS CHEMISTRIES
The CDMO Division of KD Pharma have years of experience with challenging chemistries involving toxicity, odor, industrial hygiene and environmental protection. We are experts handling these problematic chemistries and have over time made appropriate provisions to control these hazards as to work in fu...
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance