Contract Services - Analytical & Lab Services
Contract Services - Analytical & Lab Services Companies (62)
Contract Services - Analytical & Lab Services News
-
News CPHI North America a ‘one stop shop’ for professional and business development
CPHI events are renowned for connecting pharma players from across the supply chain to learn, grow, and conduct business all over the world. -
News Lonza Switzerland site to undergo expansion of microbial development capabilities
The expansion includes the installation of a pilot suite with a 50-L fermenter and automation upgrades to accelerate clinical and commercial projects -
News SGS completes expansion of its Biosafety Centre of Excellence in Glasgow
Clients to receive accelerated quality testing and reduced turnaround times -
News Recipharm expands its analytical services offering in India
The CDMO inaugurates its new analytical laboratory under Recipharm Analytical Solutions
Contract Services - Analytical & Lab Services Products (169)
-
Product Commercial Mass Spectrometry Services
Mass spectrometry is among the most powerful analytical techniques available for protein characterization. KBI’s state-of-the-art mass spectrometry core facility delivers unparalleled structural characterization services to our clients.
Our expert team brings decades of experience in protein and p...
-
Product Outsourcing
Patheon by Thermo Fisher Scientific has a broad manufacturing platform for pharmaceutical and biologic products which provides sustainable solutions for mammalian cell-based and microbial-based manufacturing, green chemistry R&D and manufacturing technologies, and finished dosage production of biopharm...
-
Product Biologic drug substance CDMO services
From pre-clinical development to commercial supply, Patheon by Thermo Fisher Scientific is an industry leader in the development and manufacture of mammalian cell culture drug substances. Patheon offers biotech and pharmaceutical companies the ability to pursue opportunities around the globe with a fully i...
-
Product SOFTGELS
At Softigel, we offer much more than traditional softgels, including everything from a single unit dosage form, to multiple delivery systems in a single dose. Softgels are an effective delivery system for oral drugs, especially those with low solubility and/or permeability (BCS Classes II, III and...
-
Product FINISHED FORMULATION , PHARMACEUTICALS
We are Manufacturing Solid/ liquid orals, Topicals, Sterile preparations (SVP/ LVP/ Eye drops ) in all therapeutic segments .
Global Pharma Healthcare has a wide range of Therapeutic segments
Cardiovascular, Oral Diabetic, Respiratory drugs, Neuro – Psychiatric, Genito – U...
-
Product Core Technologies and Services
• API / GMP Manufacturing • Rapid Process Development, Flawless Upscaling, and Economy of Scale-Production • Simulated-Moving Bed (SMB) Chromatography • Heterocyclic, Hazardous and Malodorous Chemistries • Organometallic and Cryogenic Chemistry • Transition-Metal Catalysis • High-Pressur...
-
Product API Services, Chemical Development & Manufacture
Almac’s strength in API development and manufacture is proven by being the partner of choice for many pharma and biotech companies seeking integrated drug development solutions from molecule to market.
Our technical expertise and extensive facilities enable us to offer integrated API contract ma...
-
Product Intertek Pharmaceutical Services
Intertek's pharmaceutical contract laboratory services, regulatory assistance, and supply chain assurance deliver quality and safety to meet your unique pharma and biopharma outsourcing needs. We bring quality and safety to life!
With our pharmaceutical experts working with you at every ...
-
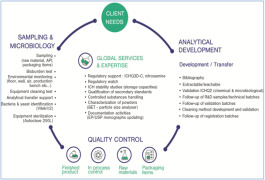
Product Analytical Services
From a drug product’s first raw materials to its release-ready batches, our experts rigorously evaluate and verify a product’s quality at key steps throughout the manufacturing process. Analytical services include material testing to verify product quality from the early stages of development, cGMP-com...
-
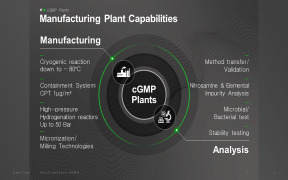
Product cGMP Manufacturing
1. Manufacturing facilities ensure: 1) Production flexibility at various scale 2) Total Production Capacity: 726 m3 including pilot production 3) Potent compound manufacturing (OEL Category 3A, CPT 1 Mug/m3) 2. Various Analytical Capabilities: 1) cGMP QC lab with HPLC, UPLC, GC, LC-MS, GC-MS, NMR, X...
-
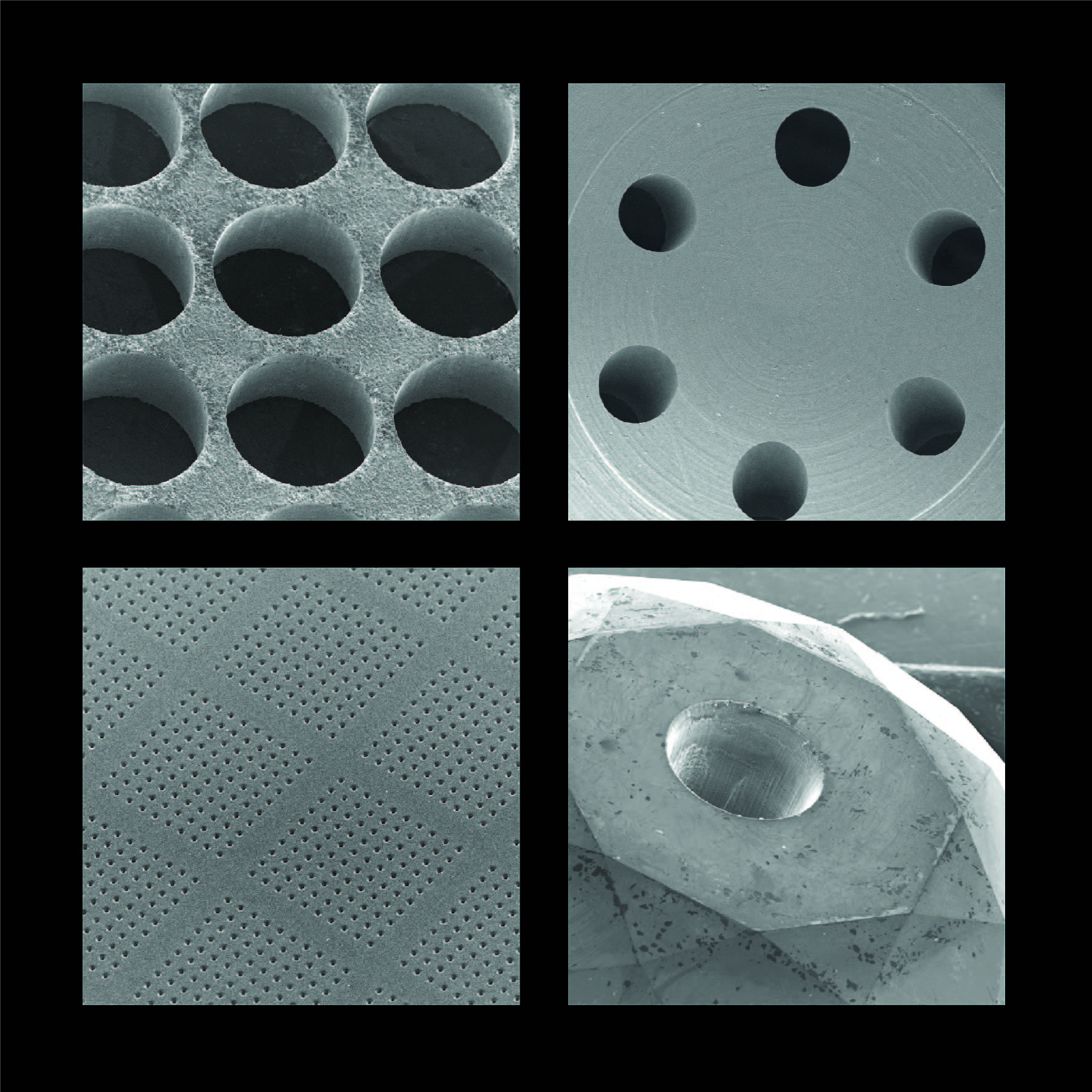
Product Laser Micromachining Services & Systems
From pinholes in stainless steel discs to complex arrays in semiconductor guide plates, we provide a wide range of ultra-high precision laser drilling, cutting, milling, scribing and ablation contract services (and systems) for numerous applications in industry & academia. With over 45 years of experie...
-
Product Services - Lab analysis
We offer the quality standards that we apply to our own environment, machines, solutions and processes as services to our customers as well.
We make continuous investments in our specialist expertise. Quality assurance is the focus of our work. To ensure that we can meet your requirements r...
-
Product QACS Pharmaceutical services
QACS Lab is GMP/GLP certified and provides contract laboratory testing for Pharmaceuticals. Methodologies stay in accordance with EMA & FDA. QACS is equipped with Microbiological - Chemical - Molecular - Packaging laboratory premises to provide variety of Pharmaceutical testing solutions &...
-
Product Mérieux NutriSciences - Pharma Services
Our services include: • Quality controls • Investigation studies • Absorption studies • R&D and validation activities • Stability & storage • Cleaning and disinfectants validation Download our brochure: https://bit.ly/48HKnVs
-
Product Analytical Services
Our Analytical Services encompass critical aspects of drug development and manufacturing, beginning with Analytical Testing to ensure product quality, employing advanced instrumentation and experienced teams to facilitate compliance. We excel in Method Development & Validation, utilizing HPLC and UHPLC...
-
Product Regulatory Testing and Research based services for Pharmaceuticals, Chemicals, Phytochemicals, Herbal Formulations, Food and Medical devices
1) Analytical Testing Of Pharmaceuticals & Cosmetics2) Biotechnological Services
3) Microbiological Services
4) Bio - Compatibility Studies of Medical Devices(As per ISO 10993USFDA and MHLW Guideline)
5) Preclinical & Toxicological Services
6) Phytochemical & AYUSH Testing Se...
-
Product In-use stability studies
We assess a variety of factors, including the effect of dilution and the dilution medium, potential adsorption to surface materials, shear forces during application, variations in extractable volume and reconstitution, formation or introduction of aggregates and (sub)visible particles, and more. While indi...
-
Product Solid & Liquid Dose Drug Manufacturing & Development
From OTC and Rx to diagnostics and dietary supplements, Avéma manufactures a full range of solid and liquid dose products, all manufactured under strict FDA guidelines and cGMP compliance. With an ever-growing portfolio of innovative formulas and a diverse mix of state-of-the-art equipment, our offerings a...
-
Product Syringe and Vial Filling for Clinical and Commerical
Lifecore’s experts have gained the know-how to build processes around your requirements for smooth scale-up and high-quality manufacturing.
Whether we’re your primary partner or a secondary source for manufacturing, we ensure you're fully satisfied with the final produ...
-
Product Impurity Synthesis
Pharmaffiliates Analytics & Synthetics (P) Ltd, is an integrated CRO (Contract Reserach Organisition) established in year 2001. Presently our group consists of more than 130 Scientists with 3 R&D Centres offering its expertise in Custom Synthesis, Impurity synthesis, Isotoped lebelled compound...
Upcoming Events
-
CPHI North America 2024
Pennsylvania Convention Center, Philadelphia
07 May 2024 - 09 May 2024 -
CPHI & PMEC China 2024
Shanghai New International Expo Center
19 - 21 June 2024 -
CPHI South East Asia 2024
Queen Sirikit National Convention Center, Bangkok, Thailand
10 Jul 2024 - 12 Jul 2024
Pharmaceutical Industry Webinars
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance






























































.jpg)

.jpg)
































.jpg)






































.jpg)




























































.jpg)



































.png)


.png)



.jpg)