Clinical Trials Phase I to IV
Clinical Trials Phase I to IV Companies (21)
Clinical Trials Phase I to IV News
-
News A Day in the Life of a Start-Up Founder and CEO
At CPHI we work to support Start-Up companies in the pharmaceutical industry and recognise the expertise and innovative angles they bring to the field. Through our Start-Up Programme we have gotten to know some of these leaders, and in this Day in the ... -
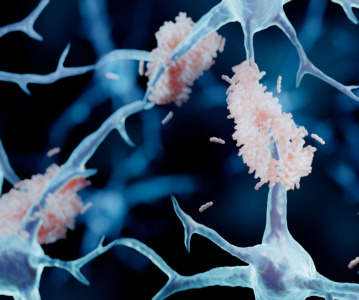
News PharmaKure gains authorisation for next stage testing on Alzheimer's treatment
Clinical stage pharmaceutical company PharmaKure gains permission from UK authorities to enter into further testing for PK051 for the treatment of patients with mild cognitive impairment associated with Alzheimer's disease. -
News CPHI Podcast Series: What does the changing US Pharma market mean for industry and patients alike?
In this week's episode of the CPHI Podcast Series Lucy Chard, Digital Editor for CPHI Online is joined by James Manser to discuss the political and market changes in the US pharma field. -
News Lexicon Pharmaceuticals gains FDA approval for heart failure drug
Lexicon Pharmaceuticals aiming to commercialise their recently approved heart failure drug in June, after receiving approval from the US FDA based on significant findings from two Phase III trials.
Clinical Trials Phase I to IV Products (40)
-
Product Clinical Research Solutions
We design and execute clinical trials so new therapies get to market in the quickest and safest way possible. Full-Service Solution: We manage and execute full-service clinical trials across major therapeutic areas within medical device, diagnostics, drug, and biologic. Functional Service Provisi...
-
Product Bioanalytical Services (GLP/GCP)
Services for Bioanalysis (GLP/GCP): In our state-of-the-art facilities, we provide GLP/GCP method development, validation, method transfer, sample analysis and pharmacokinetic and toxicokinetic support, along with automated data collection and reporting systems. Over the past 20 years, we have supported...
-
Product Small Molecule - Early Phase to Phase 2 Support
Setting the stage for the commercial process:• Route or step rebuild
• Process optimization &demonstration
• Impurities assessment & synthesis
• Full structure characterization &elucidation
• Analytical method development &qualification
• Stress studies & ICH st...
-
Product Clinical batch production
XEDEV can support clinical batch manufacturing up to Phase IIb in our clean rooms after succesfull development.

-
Product CRO services - Clinical trials with probiotics and other food supplements
Bioithas' main activity since its inception has been carrying out clinical studies in humans, both its own clinical trials and services to other companies in the pharmaceutical, biotechnology and nutritional industries.
At Bioithas, we carry out all stages of the clinical project, from trial des...
-
Product Clinical Manufacturing
.Integrated drug development service (CRO/CDMO) including clinical manufacturing and management.
The range of activities offered by Bluepharma includes:
- Clinical material cGMP manufacturing
- Clinical supply management
- clinical material
- placebo
- ...
-
Product Passionis
Passionis is a unique product specially for women, which have been related to sexual appetite boost properties, containing Liboost, Maca, Vitamin B6 and Zinc.
-
Product Clinical Manufacturing
KBI Biopharma offers a broad range of cGMP biologics manufacturing services to biopharmaceutical companies worldwide. Our capabilities include reliable manufacturing for preclinical and clinical supply.
KBI’s experienced team produces high-quality therapeutics and vaccines thro...
-
Product Clinical Supply
Recipharm have more than 20 years experience supplying clinical trial material to our clients. Our development facilities cater for a range of clinical trials from preclincal to smaller Phase III trials. We also offer commercial manufacturing for very large clinical batches.
-
Product GALENIC DEVELOPMENT
TECNALIA, experts in Pharmaceutical Development, Scale-up & Pilot Batches Manufacturing, Clinical Trials and Contract Manufacturing
GALENIC DEVELOPMENT
• Preformulation studies.
• Design and galenic develop...
-
Product Clinical Pharmacology
When you are looking for a partner who is dedicated to Phase I trials and early clinical development, rely on Quotient Sciences for the experience and expertise to seamlessly deliver your studies with the highest quality service and speed. We accelerate your molecule from first-in-human to proof-of-concept...
-
Product Antibody Drug Conjugates (ADC)
hen selecting AbbVie Contract Manufacturing, you are partnering with a leading developer and manufacturer focused on accelerating and mitigating risks to program timelines and on efficiently fast-tracking your program to completion. AbbVie’s mAb and ADC state-of-the-art facility and expert scientific team ...
-
Product Arabmed CRO
ArabMed CRO is renowned for its expertise in conducting clinical trials across all phases, from Phase I to Phase IV, with a strong track record in drug research. The company also excels in medical device clinical investigations, demonstrating its commitment to advancing medical technologies. With a focus o...
-
Product Drug Product Development
Our Vetter Development Services help biopharma customers lay a foundation of scalability, quality, and sustainable value for drug product profiles. We support drug developers in navigating key steps, decisions, and transitions in the product development cycle. This includes primary packaging evaluation, pr...
-
Product Life Science Logistics
Life Couriers provide trusted Life Science logistics support on a global scale.
Our solutions provide dependable transportation for CROs, hospitals, labs, universities and research organizations around their world, the backbone for their vital work in clinical trials, cell and gene therapy, and ...
-
Product Comparator Drugs / Reference Samples / Formulation & Whitelableing
NewLife Medicals is the preferred partner in strengthening supply chain for Innovation and R&D in Pharmaceuticals. As a dedicated pharma raw material supplier, we play a pivotal role in providing essential components for the advancement of pharmaceutical research. It is actively engaged in sourcing Ref...
-
Product Biologic drug substance CDMO services
From pre-clinical development to commercial supply, Patheon by Thermo Fisher Scientific is an industry leader in the development and manufacture of mammalian cell culture drug substances. Patheon offers biotech and pharmaceutical companies the ability to pursue opportunities around the globe with a fully i...
-
Product Early Phase Clinical Study Expertise
State-of-the-art phase I/ll unit supporting complex clinical trials • First in Human ( FIH) to Proof of Concept ( PoC) • Clinical pharmacology studies through all phases • In-house safety lab and bioanalytical services • Special patient populations (with a focus on respiratory) • Data m...
-
Product Bioequivalence studies
Intertek offers wide range of pharmaceutical services which includes bioequivalence studies. It belongs to bioanalytical services for preclinical and clinical studies category. Contact us for more information.
-
Product EU/UK Qualified Person (QP) Services and MIA license
Navigating both general and country-specific regulations and requirements to supply medicinal products to the European markets can be a complex challenge for Marketing Authorization Holders (MAH). ProPharma holds both EU and UK MIA licenses which allows us to help clients overcome the complexities of su...
Upcoming Events
-
CPHI Middle East 2024
Riyadh Front Exhibition & Convention Center Riyadh, Saudi Arabia
10 Dec 2024 - 12 Dec 2024 -
Pharmapack Europe 2025
Paris Expo, Porte de Versailles - Hall 7.2 | Paris, France
22 Jan 2025 - 23 Jan 2025 -
CPHI Japan 2025
East Halls 4, 5 & 6, Tokyo Big Sight, Tokyo, Japan
09 Apr 2025 – 11 Apr 2025
Pharmaceutical Industry Webinars
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance



































































.jpg)



.png)







.png)

.png)



.jpg)


