All News
All news
-
News New research highlights lack of confidence in serialization preparations
40% of industry professionals admit they are not clear about the global requirements for serialization. -
News STA signs 5-year supply agreement with TESARO
STA will provide supplies of certain starting and intermediate materials for the recently launched Zejula. -
News Merck launches Remicade biosimilar for all eligible indications, in the US
The biosimilar's list price will represent a 35% discount to the current list price of Remicade. -
News Long-acting injection to remove daily dosing burden for HIV patients?
Study results showed that long-acting cabotegravir and rilpivirine maintained viral suppression. -
News Lilly backs Nektar Therapeutics' novel immunological therapy
NKTR-358 has the potential to treat a number of autoimmune and other chronic inflammatory conditions. -
News Novartis receives positive CHMP opinion for Rydapt
If approved, Rydapt would represent the first targeted treatment for newly diagnosed FLT3-mutated AML in the EU. -
News GSK receives FDA approval for a new self-injectable formulation of Benlysta
The approval marks the first subcutaneous self-injection treatment option for patients with systemic lupus erythematosus. -
News Boehringer Ingelheim breaks ground on $217 million expansion of Fremont, California manufacturing facility
Expansion will triple capacity, add nearly 300 new jobs. -
News Merck and Pfizer collaborate with Corning to modernize pharmaceutical glass packaging
Collaborations result in development of Corning Valor Glass. -
News Biohit Acetium® lozenge is a highly effective means to stop smoking – results confirmed in a new large-scale trial
The second smoking intervention study started in 2016 with Biohit Acetium lozenge has been completed confirming the efficacy of Acetium lozenge as an effective way to assist in smoking cessation. This new trial was designed to be adequately powered ... -
News NEWGUARD: SAFER PREFILLED SYRINGES & FEWER CHANGES
Given the rising number of guidelines to reduce risks of needlestick injuries, pharmaceutical companies have been searching for systems to ensure safe syringe use. Despite great innovations, adopting new safety systems has always been a trade-off betwe... -
News Recipharm and LIDDS establish industrial manufacturing capabilities for a novel prostate cancer product
GMP Manufacturing line industrialised according to a unique process invented by LIDDS, involving the installation of novel equipment that is new to the pharmaceutical industry. -
News Catalent and academia on quest to better understand pediatric drug formulation and delivery challenges
Goal of the collaboration is to identify therapies and diseases for which there is a high need for pediatric-friendly formulations. -
News LifeArc negotiates exclusive licence deal with Nemysis for oral iron supplement technology
IHAT, a novel synthetic material, is a commercially viable alternative to ferrous salts for iron supplementation. -
News Positive results from 12-month Phase III safety study of Revefenacin in patients with COPD
These data, combined with positive results from two Phase III efficacy studies, support NDA filing planned for fourth quarter of 2017. -
News COVIS TAPS VERIFY AHEAD OF THE EXTENDED DSCSA MANDATE
This month in Outsource Pharma online, Michael Howe, CEO of Verify Brand, a Minneapolis-based serialization software provider, addresses that even though the DSCSA deadline was extended, many pharma manufacturers, such as Verify Brand’s client Cov... -
News * NEW * PRESERVATIVE FREE CROSSED LINKED HYALURONIC ACID DROPS
World first preservative-free eye drops with cross-linked Hyaluronic AcidCross-linked Hyaluronic Acid is a form of Hyaluronic Acid more stable and effective which increases:- Moisturizing properties - Long-term effectiveness- Viscosity and physica... -
News Onyx helps to accelerate TopiVert’s compounds to clinic
With support from the CRO, TopiVert has been able to accelerate scale-up and produce GMP batches, which are now being used in clinical trials. -
News GSK delivers double-edged sword
Company announces investments, the sale of several products and a proposal to close a UK manufacturing site. -
News South Korean pharma rapidly becoming manufacturing export hub
Overseas buyers at CPHI Korea double in the past 2 years. -
News VIALS MACHINERIES
TWO MORE VIALS MAKING MACHINERIES, FOR AN INCREASED MANUFACTURING CAPACITY -
News Ordesa Catalogue
ordesa corporative brochure -
News ordesa catalogue
ordesa corporative brochure -
News Transfer technology expert expands US presence following multi-million-pound investment
Investment follows demand for the company's AseptiSafe and PharmaSafe product ranges, which transfer APIs in small- and large-scale pharmaceutical manufacturing environments. -
News FDA approves new treatment to reduce the risk of breast cancer returning
Nerlynx is the first extended adjuvant therapy for patients with HER2-positive breast cancer. -
News Shire expands broad monoclonal antibody research platform
Shire obtains worldwide license to pursue development and commercialization of a novel, potentially differentiated, pre-clinical bi-specific antibody candidate for Hemophilia A. -
News Amgen and Array BioPharma announce preclinical license and collaboration agreement in inflammation
Array to advance preclinical program for autoimmune disorders; Amgen responsible for clinical development and worldwide commercialization. -
News Technology adoption fuels growth for pharma tech firm
Company has experienced double digit growth across its automation, MES and managed services offering. -
News Catalent launches new OptiForm Solution Suite
The service offers quick and efficient path from late-stage discovery to Phase I clinical trials. -
News FDA approves Impax's AB rated generic Concerta Extended-Release Tablets CII
Company expects to launch by the end of the year. -
News Alzheon presents new data for ALZ-801 on novel MOA and long-term clinical efficacy at the AAIC
Presentations highlight recent discovery of inhibition of formation of toxic beta amyloid oligomers and new insights for Precision Medicine approach for ALZ-801 targeting APOE4 carriers. -
News Ground-breaking preclinical data to be presented at AAIC 2017
KalGene’s Alzheimer’s therapeutics candidate, licensed from the NRC (KAL-ABP-BBB), crosses the blood-brain barrier, and reduces amyloid-beta burden in a transgenic rat model after a 4-week treatment. -
News FDA committee recommends approval of Mylan and Biocon's proposed biosimilar trastuzumab
Vote marks first proposed biosimilar trastuzumab to be recommended by the committee. -
News GSK ships 2017-18 seasonal influenza vaccines for US market
Company expects to supply up to 40 million doses across both vaccines for the US market in the 2017-18 season. -
News Novartis confirms 5-year data for Cosentyx reinforcing sustained efficacy and safety profile in psoriasis
Cosentyx continues to demonstrate it can provide what psoriasis patients want - a life with clear skin. -
News Ascendis Health latest acquisition
Ascendis Health recently declared the acquisitions of Sunwave Pharma SRL and NHP Natural Health Pharma Ltd unconditional, together with Cipla Agrimed Proprietary Limited and Cipla Vet Proprietary Limited. -
News Bosch sets standards in sterile powder filling with AFG 5000
The system's new, variable transport system avoids bottlesnecks or delays in workflow. -
News Novartis CAR-T cell therapy CTL019 recommended for FDA approval
A BLA for this indication is under FDA priority review; if approved, CTL019 could become first CAR-T cell therapy available. -
News FDA accepts sNDA for Xeljanz for the treatment of adult patients with active ulcerative colitis
Anticipated Prescription Drug User Fee Act (PDUFA) action date for the sNDA is March 2018 . -
News Merck KGaA refines Western European life science production site network
Company to invest €90 million and cut 200 jobs. -
News Lilly reaches settlement agreement in US Cialis patent litigation
Cialis exclusivity is now expected to end at the earliest on 27 September 2018. -
News FDA approves Blincyto to treat relapsed or refractory B-cell precursor acute lymphoblastic leukemia
Blincyto is the first and only Bispecific T cell Engager (BiTE) immunotherapy to demonstrate superior overall survival versus standard of care chemotherapy. -
News SMC says ‘yes’ to Opdivo for blood cancer patients
Patients in Scotland will now join those in England and Wales and be able to benefit from this potentially life-extending immunotherapy on the NHS. -
News Erytech collaborates with Queen’s University to advance product candidate for rare metabolic disorders
The collaboration will leverage the expertise of Queen’s University and Erytech’s ERYCAPS platform technology. -
News CPHI Worldwide 2017
CPHI Worldwide 2017 - Messe Frankfurt, Germany (24 - 26 October 2017)Visit us at: 110B12 -
News Serialization and Tamper Evidence
Please see further information on "Serialization and Tamper Evidence" at KLOCKE on the attached pdf-file. -
News Sanofi to acquire Protein Sciences
Acquisition adds recombinant-based influenza vaccine to Sanofi Pasteur's portfolio. -
News New data reinforce the effectiveness of Viberzi to treat the symptoms associated with IBS-D
Viberzi proven safe and effective in patients who reported inadequate response to prior loperamide use. -
News Daiichi Sankyo, Max Planck Innovation and Lead Discovery Center announce cancer research collaboration
Parties to optimize certain novel compounds that target cancer cell transcription and proliferation. -
News Agilent Technologies acquires Raman spectroscopy innovator, Cobalt Light Systems
Cobalt's portfolio of solutions accelerates entry into high-growth Raman spectroscopy market and expands Agilent’s value proposition for pharmaceutical and biopharma customers. -
News Trial to identify breath biomarkers to improve the early detection and diagnosis of cancer
Owlstone Medical and Cancer Research UK initiate pan cancer clinical trial. -
News BMS's Orencia receives FDA approval for treatment of active psoriatic arthritis in adults
Orencia now approved in three autoimmune diseases. -
News CPHI Worldwide opens entries for largest ever Pharma Awards
The 2017 awards include eight new categories - celebrating outstanding achievements in the pharma industry. -
News Disappointment for Scottish patients with PAH as SMC says ‘no’ to Uptravi
Selexipag is an innovative, oral treatment that specifically targets the prostacyclin pathway. -
News Information sharing - the most valuable benefit expected from serialization?
NEXUS 17 attendees claim the greatest serialization challenge thus far has been line vendors’ inability to deliver a solution on time and within scope. -
News UK Supreme Court rules in Lilly's favour on Alimta vitamin regimen patents
Actavis's products directly infringe Lilly's vitamin regimen patents in the UK, France, Italy and Spain. -
News Shire submits IND application to FDA for gene therapy candidate SHP654 for treatment of hemophilia A
SHP654 aims to deliver sustained protection against bleeds for patients with hemophilia A. -
News Almac Discovery granted Orphan Drug Designation for ALM201 programme in ovarian cancer
ALM201 is a therapeutic peptide developed to mimic some of the properties of the naturally occurring protein FKBPL. -
News DRUGCOAT..The polymer of choice in functional coating
To know in brief about the concept development of tablet coating system, transformation in tablet coating system, its uses, various opportunities with acrylate polymer advantage. -
News ChargePoint Technology invests in production capabilities for large-scale containment valves
The new 5-axis machine will speed up the production and delivery of larger diameter valves up to 350 mm. -
News I Holland Presents Interphex Japan with its innovative tooling solutions
IH-TMS Tool Management System and the company's latest tool coating solutions interest exhibition visitors. -
News Lonza completes acquisition of Capsugel
Acquisition create leading integrated solutions provider to the global pharma and consumer healthcare industries. -
News Impax launches additional strengths of generic Focalin XR extended-release capsules CII
Company has immediately initiated commercialization activities. -
News Novartis receives EU approval for Cosentyx label update
Label update includes 52 week data from CLEAR study demonstrating long-term superiority of Cosentyx versus Stelara in psoriasis. -
News Specialist CRO expands pharmacovigilance capabilities
Pharma clients now have direct and immediate access to a fully compliant safety database without needing to invest in their own system or use an interim solution to ensure compliance. -
News Phase III study evaluating safety and efficacy of adjuvant Opdivo in resected melanoma patients meets primary endpoint
Opdivo demonstrates superior recurrence-free survival versus Yervoy in adjuvant setting in CheckMate -238. -
News New design for nutrients - Doppelherz sparkles with effervescent IML packaging from Sanner
Brilliance tubes provide a user-friendly and attractive all-in-one solution. -
News ChargePoint Technology invests in production capabilities for large scale containment valves
ChargePoint Technology has invested in new manufacturing machinery at its UK facility to meet increased demand for larger diameter valves for use in large scale drug manufacturing that requires containment. -
News New Operational Flexible Serialization Suite at Hay-on-Wye
PCI Pharma Services (PCI) are pleased to announce a significant expansion of our market-leading Serialization capability at our Hay-on-Wye site in the UK. -
News BIOCAD’s rituximab biosimilar to receive MA in India
First shipment of BIOCAD’s Acellbia to India is scheduled for September 2017. -
News First patients treated in Phase I/II trial of NOX-A12 combined with Keytruda in metastatic pancreatic and colorectal cancer
NOX-A12 has the potential to transform cancer types that are resistant to checkpoint inhibitor therapy into checkpoint inhibitor sensitive tumours -
News Sun Pharma & Samsung BioLogics announce strategic manufacturing tie-up for Tildrakizumab
Tildrakizumab is an investigational IL-23p19 inhibitor being evaluated for the treatment of moderate to severe plaque psoriasis. -
News Idifarma approved for EU-GMP commercial capsule manufacturing
Idifarma, a Spanish CDMO, has expanded its GMP manufacturing offering with the installation of new automatic capsule filling capabilities at its EU-GMP approved plant in Pamplona, Spain. -
News Common antimicrobials help patients recover from MRSA abscesses
NIAID-funded trial counters current thinking about treatment effectiveness. -
News Lilly's Olumiant gets the thumbs up in Japan for the treatment of rheumatoid arthritis
In clinical studies, baricitinib has demonstrated significant improvement in the signs and symptoms of RA compared to standard-of-care therapies. -
News New second generation solvent evaporators from Biotage
Three new TurboVap evaporation systems increase laboratory reliability. -
News FDA approves Vectibix for use in wild-type RAS metastatic colorectal cancer
First-and-only fully human monoclonal anti-epidermal growth factor receptor antibody approved by the FDA for this patient population. -
News Gyros Protein Technologies introduces new ADA solution for immunogenicity market
Developed to support assay development and validation for detection of anti-drug antibodies. -
News Exscientia enters strategic drug discovery collaboration with GSK
Pre-clinical collaboration focused on up to 10 targets nominated by GSK. -
News Patheon to invest $45 million, expanding capabilities to support continued growth
Investments in commercial and development spray drying, and flexible manufacturing solutions. -
News Tailor-made Flexible Packaging Materials for the Pharmaceutical Industry
Danapak Flexibles A/S, a subsidiary of Schur Flexibles Group, has been a supplier of flexible packaging materials for almost 40 years. -
News WHO WE ARE? COME AND MEET US
Our Management -
News Senn Chemicals - Solutions Synthesized PPT
Senn Chemicals - Solutions Synthesized PPT -
News Bioverativ completes acquisition of True North Therapeutics
Strengthens leadership in rare blood disorders with first-in-class candidate to treat cold agglutinin disease, a chronic autoimmune hemolytic anemia with no approved therapies. -
News Top seller of Global Phenacetin Market 2017
The Global Phenacetin Market 2017 examines the performance of the Phenacetin market. It encloses an in-depth judgment of the Phenacetin market state and the competitive landscape globally. This report analyzes the potential of Phenacetin mark... -
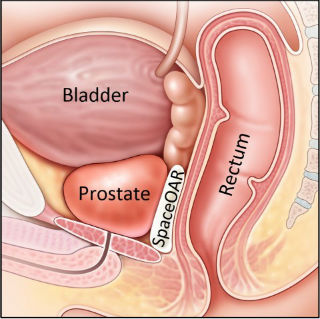
News First prostate cancer patients treated with Augmenix's SpaceOAR Hydrogel in Israel
The Chaim Sheba Medical Center now offers the hydrogel spacer to reduce side-effects during prostate cancer radiation therapy. -
News Copley Scientific releases upgraded SPU 2000 for reduced inhaler testing variability
Company's modification makes the SPU 2000 a more cost effective laboratory tool. -
News Novartis receives EU approval for first-line use of Zykadia in ALK-positive advanced NSCLC
In ALK-positive advanced NSCLC patients, Zykadia demonstrated superior median progression-free survival compared with SOC chemotherapy with maintenance. -
News Researchers develop microneedle patch for flu vaccination
The patch can dramatically reduce the cost of vaccination, as self-administration can eliminate the need to have health workers oversee the process. -
News BASF plans significant investment in ibuprofen capacities in Germany and North America
New world-scale ibuprofen production plant in Ludwigshafen; additional capacity expansion of ibuprofen plant in Bishop, Texas. -
News UK availability of Rixathon and Erelzi paves way for NHS savings
Two new biosimilar medicines available to treat patients with specific blood cancers and a range of inflammatory condition. -
News Merck's anacetrapib meets primary endpoint
Company to consider whether to file new drug applications with the FDA and other regulatory agencies. -
News Vectura enters new development and licence agreement for a US inhaled generic
Agreement concerns an existing inhaled combination therapy for asthma and COPD delivered using a pMDI. -
News INTERVIEW ON BIOCORP’S CONNECTED MEDICAL DEVICES
In this interview, Mr Guillet discusses Biocorp’s existing connected device offering, how it delivers value for the company’s pharmaceutical partners, the development of the accompanying software and data architecture, and how Biocorp has very signific... -
News Amino Acid Building Blocks
Amino Acid Building Blocks Flyer -
News LifeArc, Dstl and CDRD collaborate to identify antibacterial drug targets
Novel approach capitalises on commonality across pathogens. -
News Allergan introduces REFRESH OPTIVE MEGA-3 enhanced with flaxseed oil
New offering restores lipid layer and brings hydration to all three layers of tear film damaged by Dry Eye. -
News Bioconjugation specialist Innova Biosciences acquired by SYGNIS
Innova’s range of antibody and protein labeling products and services ideally complements SYGNIS’ customer base and existing genomics and proteomics portfolio. -
News QS Pharma receives approval from EMA for commercial manufacture
The CDMO is now approved to manufacture medicinal products for the US, Europe and Japan. -
News B&W Tek Releases Updates to BWSpec® Software with Version 4.10
B&W Tek, a mobile spectroscopy solutions company that delivers lab quality Raman, LIBS, UV-Vis and NIR solutions through user-friendly mobile platforms, is proud to announce updates to their BWSpec software, expanding the software supporting B&... -
News Technology selection identified as biggest hurdle in serialization race for compliance
Resourcing the deployment of serialization systems and processes also cited as a significant barrier to compliance with European FMD, -
News Sartorius Stedim Biotech launches Active Dashboard 2
Advanced software solution ensures superior manufacturing quality and enables effective decision-making. -
News Hikma Pharmaceuticals makes leadership and organisational changes to US business
US business will be organized into two entities - a US Generics Division and a US Injectables Division. -
News Takeda and Biological E. partner to develop low-cost combination vaccines for low- and middle-Income countries around the globe
Two recently-signed agreements will transfer Takeda’s measles and acellular pertussis vaccine technologies to India-based multi-national company Biological E. Limited to develop low-cost combination vaccines including diphtheria, tetanus and acellular ... -
News Cladribine Tablets receives positive CHMP opinion for treatment of relapsing forms of MS
Cladribine Tablets is the first and only investigational medicinal product to have shown a sustained 4 years of disease control with a maximum of 20 days of oral treatment over 2 years in clinical trials. -
News Pfizer receives expanded Health Canada approval for Ibrance in HR+, HER2- metastatic breast cancer
New indication supported by results of Phase III PALOMA-3 Trial of Ibrance in combination with fulvestrant. -
News Sartorius Stedim Biotech introduces Sartobind cassettes
New modular membrane chromatography solution for large-scale applications . -
News Biogen’s Imraldi, an adalimumab biosimilar candidate referencing Humira, granted positive opinion by CHMP
If approved, Imraldi will be the third anti-TNF biosimilar in Biogen’s portfolio in Europe. -
News Juniper Pharma Services brings PSD-1 spray dryer online
The addition enables the CDMO to scale-up early-phase bench top processes to support later stage clinical development and niche, low volume manufacturing requirements. -
News Aptar Pharma holds its first dermal drug delivery seminar in China
Attendees learnt how Aptar's solutions could be applied to the Chinese market in a changing regulatory environment. -
News FDA approves Mydayis – a once-daily option for ADHD symptom control in patients 13 years plus
Mydayis demonstrated improvements lasting up to 16 hours post-dose, beginning at 2 or 4 hours post-administration, compared with placebo, in total score on a skill-adjusted math test that measures attention in ADHD. -
News Arcinova investment reduces R&D and manufacturing time by more than a month
Arcinova has invested in a Uniqsis FlowSyn continuous flow reactor enabling them to develop and simplify the scale-up of a challenging API batch process. -
News Novartis achieves regulatory milestone for AMG 334 in migraine prevention with EMA filing acceptance
AMG 334 (erenumab) is the first anti-CGRP monoclonal antibody developed for migraine prevention to receive EMA regulatory filing acceptance. -
News Accelerating freeze drying cycle development with just seven vials
Compared with other small-scale R&D freeze dryers, the LyoCapsule lyophilizer is the only system that can monitor and independently control the wall temperature via use of the center vial product temperature. -
News The “BB Dose”: a Unither innovation that guarantees more safety with the infant
Recently, Unither Pharmaceuticals partnered with Biolane to developed a single-unit dose featuring a more rounded tip that fits perfectly with babys’ nostrils. The “BB dose” guarantees a greater safety of the gesture of the mother with the infant. ... -
News Takeda completes new manufacturing facilities at the Oranienburg site in Germany
The manufacture of solid dosage form pharmaceutical products would be transferred from the Osaka Plant to the new plant. -
News QuVa Pharma announces availability of sodium bicarbonate syringe
Compounded Sodium Bicarbonate PF 8.4% 50mEq per syringe is expected to help alleviate chronic drug shortage impacting the nation's hospitals. -
News Glenmark Pharmaceuticals licenses small molecule oncology compound from APC Therapeutics to expand IO portfolio
The compound has the potential to be used as a monotherapy or in combination with approved therapies to address unmet needs in cancer treatment. -
News Merck Ventures creates new immuno-oncology company iOnctura
iOnctura is set up to develop a pipeline of assets targeting immunosuppression in the tumour microenvironment. -
News FDA accepts Amgen's sBLA to expand indication for Xgeva to include multiple myeloma patients
Xgeva is indicated for the prevention of skeletal-related events in patients with bone metastases from solid tumours and is the number one prescribed agent by oncologists for this indication in the US.
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance