All News
All news
-
News Oxford Genetics signs major supply and licensing agreement for CRISPR engineered mammalian cell lines
Company moves away from manual processing in favour of automated, scalable platforms. -
News BASF receives award for its portfolio of natural cosmetic ingredients
Company recognised for its innovation and continual delivery of new natural ingredients. -
News A new era for migraine patients
EU approves Novartis's Aimovig, a first-of-its-kind treatment specifically designed for migraine prevention. -
News Extended distribution agreement enables customers to continuously improve their bioprocesses
Agreement includes Pall's next-generation Kaneka KanCapA 3G sorbent for the primary capture of mAbs from clarified cell culture. -
News Breast cancer device receives FDA approval
The first non-radioactive, dual-tracer for sentinel lymph node biopsy approved in the US. -
News Amgen breaks ground on next-generation biomanufacturing plant in Rhode Island
The plant will be first-of-its-kind in the US. -
News Cherwell publishes guide on environmental monitoring processes and validation
Supporting EM programs in preparation for proposed EU GMP Annex 1 changes. -
News Building contingency plans for an ambiguous post-Brexit market
One company's positive approach to Brexit preparations, despite the lack of definition. -
News Solving costly tablet manufacturing issues
I Holland to share their 70 years' experience in an ‘ultimate’ tooling seminar. -
News Post-Brexit medicines - stuck in the UK?
The EMA needs to provide more action and guidance to help UK pharmaceutical manufacturers, says Thomas Beck, senior vice president, quality management, Recipharm. -
News TTP completes expansion of life sciences lab facility for Desktop Biology programmes
Dedicated facility developed in response to increasing demand for product development projects in diagnostics, R&D and bioprocess. -
News Partnership struck to aid bio manufacturers
Pall and BioSciencesCorp unite to provide "total solutions" for today's drug manufacturers. -
News RisdropTM, Watch the video
Risdrop, our innovative user-independent eye dropper, helps reduce the risk of overdosage by delivering the same drop every time irrespective of squeeze force and inclination. -
News WuXi STA’s Jinshan site passes fourth FDA inspection
First CDMO in China that is approved to supply commercial APIs for innovative drugs by regulatory agencies in the US, China, EU, Canada, Switzerland, Australia, and New Zealand. -
News WuXi STA and Antengene sign development and manufacturing agreement
WuXi STA chosen for its end-to-end CMC platform for new drug development. -
News Pharmacovigilance post-Brexit - simpler than previously thought?
To meet new PV regulations, the industry has to face a "multitude of unknowns and an ever-moving target". -
News Filling and closing machine from Bosch honored with Red Dot Award
The AGF 5000 is a machine with a compact and space-saving design, easy handling and a small number of format parts. -
News New screening company exploits the latest advances in microfluidics and 3D culture
The ability to micro size drug-cell interactions will allow pharmaceutical and biotech companies to do 100x more testing for the same money spent as well as increasing productivity. -
News ProBioGen and Pionyr initiate 2nd immuno-oncology contract development and manufacturing project
Parallel cell line development and analysis will allow for optimal cell line selection while compressing the development timeline. -
News FDA approves first smallpox drug
Action ensures that the US is prepared should smallpox be used as a bioweapon. -
News South Korea's finished drug market growth driven by generics
FDF launches at CPHI Korea as country set to become regional hub. -
News Wasdell Group launches serialisation service
Company will offer compliant products to all markets, including Europe and the US. -
News Natural lipid acts as potent anti-inflammatory
Scientists see therapeutic potential against bacteria, viruses. -
News Stability studies trends
Dr Ramesh Jagadeesan, Sr. Director - Analytical Development at Recipharm, speaks to CPHI Online on the trends and challenges involved with conducting stability studies for firms that supply to multiple countries, and the advantages of using a contracto... -
News CPHI Worldwide opens entries for 15th Annual Pharma Awards
Winners will be announced live at the Gala Ceremony in Madrid with over 500 of Pharma’s top executives. -
News Recipharm launches standalone serialisation service
Company will be able to add 2D codes, human-readable text and tamper evidence to pre-packed medicines using existing equipment across its facilities. -
News Bosch to sell its packaging business
Packaging technology is not a core Bosch business. -
News Insights in lyophilization science
The latest developments in process development, nucleation control, scale-up of freeze drying cycles and development in lyophilization technology. -
News Plasticell leads gene therapy manufacturing consortium
Consortium to develop advanced technologies for the manufacturing of ex vivo gene therapies. -
News I Holland woos exhibition visitors with new tabletting technologies
Company's Wear Indicator Layer attracts the most attention. -
News Forté Pharma strengthens its leadership in weight control in France
Company consolidated as one of the leading laboratories in weight control thanks to the development of its existing range and new launches. -
News NSF’s Peter Gough Presents on Brexit Impact at 2018 Pharmaceutical Industry Network Group
Peter Gough, NSF International’s Executive Director of Pharma Biotech, provided a practical overview of the regulatory implications of Brexit at the 2018 Pharmaceutical Industry Network Group (PING) seminar, held June 8, 2018 in Hertfordshire, UK. -
News Follicum develops new direct-to-scalp formulation
The topical treatment for hair loss boasts a 'commercial' formulation. -
News Ingredients to improve bioprocess manufacturing efficiency
Experts warn bio industry needs to do more to create a sustainable pipeline of talent. -
News Lilly wins patent case over Alimta
Ruling prevents Dr Reddy's Laboratories will from launching their alternative salt forms of pemetrexed until the patent expires. -
News First FDA approval of marijuana-based drug
Approval for seizures associated with Lennox-Gastaut Syndrome or Dravet Syndrome, two rare, severe childhood-onset epilepsies. -
News Juniper Pharma Services expands laboratory facilities to meet with growing demand
Nottingham, UK – Juniper Pharma Services (JPS), a Contract Development and Manufacturing Organisation (CDMO) specialising in the development of challenging small molecules, has announced a significant expansion to its UK-based drug development and clin... -
News Introducing Ascendis Health
Ascendis Health is a South African-based international health care company marketing leading brands and products. Founded in 2008 and listed in the healthcare sector on the JSE in November 2013, the group has complemented its organic growth b... -
News Patch-in-a-Can technology to delivery pain management product
MedPharm licenses its MedSpray ‘Patch-in-a-Can’ technology to Virpax Pharmaceuticals. -
News CellGenix completes first step of facility expansion
Next expansion step will increase finished product capacity for recombinant proteins by more than tenfold. -
News Solvent removal for kilo-scale preparative chromatography
The Rocket 4D Synergy uses proprietary vacuum technology to suppress solvent bumping and foaming. -
News Lyogistics Zero: an innovative freeze dryer vial automatic loading/unloading system with the ability to have CIP/SIP inside chamber
Breakthrough innovations in Freeze Drying Lyogistics Zero is the only vial automatic loading & unloading system for freeze-drying processes which can be cleaned (CIP) and sterilized (SIP) in place, inside the freeze-dryer chamber. -
News Collaboration to provide GMP-based services
Researchers to benefit by receiving a rapid and cost-effective scaled supply of new compounds. -
News Recipharm expands inhalation capabilities with acquisition of Sanofi CMO business located in UK
Acquired business will enhance Recipharm’s scale and provide access to new customers and higher margin specialised technologies. -
News Extended co-operation agreement to benefit Sartorius customers
Agreement will enable customers to achieve easier scalability of processes ranging from lab-scale to commercial-scale production of biopharmaceuticals. -
News Attendance rise sees CPHI & P-MEC India 2018 move to Indian capital
The India Expo Mart brings the event closer to Indian regulatory and legislative hub and will encourage international audiences. -
News Innovative coating process for tablet manufacturers
The Wear Indicator Layer is expected to have a "huge impact" on customers' production. -
News TraceLink customers receive EMVO approval for data submission to EU Hub
Company has developed a standard feature for its customers to execute the EMVO conformance testing successfully. -
News Locate Therapeutics secures £2 million to fast-track development of innovative cell therapy programs
TAOS matrix system and products hailed as potential ‘game changers’ in the field of regenerative medicine. -
News Process innovation needs to be undertaken in early R&D phase
Much needed more efficient manufacturing processes for ADCs aren't being completed by pharma. -
News Recipharm invests in Brexit preparations
‘Brexit taskforce’ will see Recipharm prioritise its preparations and invest in the necessary capabilities and equipment to ensure seamless operations post March 2019. -
News New finished product manufacturing hub in the Middle East
Forgotten markets of Africa and Arabia to see increased growth fuelled by international and domestic partnerships and imported APIs. -
News Amneal to close it Hayward, California-based facilities and operations
Company to streamline operations and capture cost synergies. -
News Characterisation technologies for pharma manufacturers
Testa Anayltical to discuss their latest developments and application advances in Light Scattering technology, GPC/SEC instrumentation and Zeta potential measurement. -
News Solid dosage CDMO invests in spray drying
New equipment allows Genvion to take customers from development through to commercial manufacture. -
News Tjoapack wins packaging contract for bladder cancer detection products
Tjoapack to perform the secondary packaging and assembly of product kits for supply to 25 European countries, the US, Canada and Australia. -
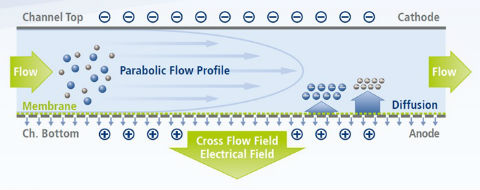
News Comparative analysis of a complex monoclonal antibody
New poster describes how the EAF4 module enabled simultaneous measurement of size and surface charge characteristics of antibodies and proteins. -
News The shortest way from the lab to continuous production
Bosch's new Xelum R&D for continuous production of oral solid dosage forms ensures a short time-to-market and optimum dosing of APIs. -
News Internationalisation and regulatory reform driving growth and investment at CPHI China
2018 event prepares for surge in international attendees as MAH nears national rollout. -
News Dipharma Francis S.r.l. completes acquisition of Kalexsyn, Inc.
Milan, Italy – Dipharma Francis S.r.l., (Dipharma), a leading European manufacturer of Active Pharmaceutical Ingredients, is pleased to communicate that it has completed its previously announced acquisition of Kalexsyn, Inc. (Kalexsyn), a world-... -
News Investing in blistering capabilities to boost flexibility
Postponement printing is presenting interesting benefits for the pharmaceutical supply chain. -
News Thermo invests $50 million to enhance biologics capabilities
With the capacity expansion, Thermo Fisher will have the second largest base of single-use bioproduction capacity at a CDMO in the world. -
News Lilly expands oncology pipeline with acquisition
Acquisition of AurKa Pharma expands Lilly's oncology pipeline with early-phase asset being studied in multiple tumour types. -
News Serialisation — looking for value-added opportunities
Recipharm's Staffan Widengren, Director Corporate Projects, discusses why CMOs should be focusing on the value-added opportunities presented by serialisation. -
News Increase performance and production
In order to ensure a new manufacturing contract with Merck SA, Grupo Azevedos, an international pharmaceutical group, has invested 2M euros approx. in their own plant unit Sofarimex with a new Groninger high-speed liquid filling line.
qq... -
News Versatile lyophilizer for research, development or small-scale production
Allows for easy and intuitive scale-up from research. -
News MOEHS NEW RESEARCH CENTER
Moehs group built a New Research Center, A new state-of-the-art 2.762sqm facility. Full capacity and expert team for your projects.
-
News New online customer portal for custom research services
Customers to gain a greater insight into their projects, better track project progress and improve communications. -
News First-of-its-kind Smart monitoring technology launches
Managing the risks associated with Industrial Hygiene and Quality and meeting operational efficiency targets. -
News Keeping a close eye on processes and lines
New Industry 4.0 solutions from Bosch for more transparency. -
News Ardena acquisition strengthens API offering
Acquisition enables Ardena to manufacture batch sizes up to 100 kg. -
News Business combination creates 5th largest generics business in the US
Anmeal and Impax create diversified pharmaceutical company. -
News WuXi STA Changzhou site passes first FDA inspection
The integrated R&D and manufacturing facility is expecting more products to go into commercial production post approval. -
News Essential malaria parasite genes revealed
NIAID-funded research could aid antimalarial drug development. -
News Value-added formulations for generic and branded pharma manufacturers
Noramco and SPI Pharma sign agreement to develop ready-to-implement, patient-friendly formulations for pharmaceutical manufacturers. -
News WuXi STA to build new R&D center in Shanghai
Integrated one-site solution for partners to push forward innovative APIs and advanced intermediates from preclinical to commercial stage. -
News New lyophilization system to stabilize thermolabile and delicate active ingredients
Energy efficient, short cycle times and reliable product quality in large batches. -
News New display and Operation Concept for the ALPINE Air Jet Sieve e200LS
Retrofitting made easy
New Display and Operating Concept for the ALPINE Air Jet Sieve e200 LS
The latest air jet sieve in the family of ALPINE's air jet sieves e200 LS is the device for analysing the part... -
News See how I Holland develops tablets to prevent common problems
Company to bring its new services and products into the spotlight at ACHEMA. -
News Sterling Pharma Solutions experiences record US growth
The CDMO's US sales have increased from £15.5 million to £41 million since 2016. -
News Cost-effective, scalable process development of vaccines in cell culture
Sartorius Stedim Biotech introduces its mini microcarrier bioreactor for culturing adherent cells. -
News Dipharma Francis S.r.l. enters into agreement to acquire Kalexsyn Inc.
Dipharma Francis S.r.l. (Dipharma), a leading European manufacturer of Active Pharmaceutical Ingredients, and Kalexsyn, Inc. (Kalexsyn), a world-class Contract Research Organization (CRO) providing chemistry services to the biotechnology and pharmaceut... -
News Sterling invests £6m in pilot plant facility
Investment enhances the CDMO's scale-up and small to midscale commercial API manufacturing capabilities. -
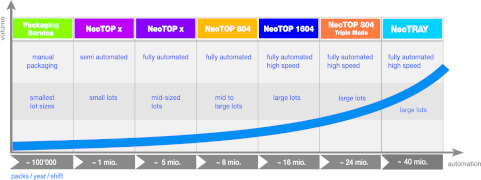
News Dividella ACCELERATES high-output packaging with new NeoTRAY flagship cartoner
Grabs, Switzerland: – Top-loading parenteral and secondary packaging specialist Dividella has set a new benchmark for high speed parenteral packaging with its latest NeoTRAY fully automatic family of cartoners that supports outputs up to 40 million... -
News Wacker acquisition increases cost-effective pharmaceutical production
The contract manufacturer strengthens its pharmaceutical-protein business with Dutch acquisition -
News BASF drives sustainable palm with major portfolio switch
First customers have implemented portfolio changes. -
News Manufacturing collaboration to advance PMD-026 toward IND
MD-026 is the first RSK inhibitor to demonstrate potential disease-modifying properties in triple-negative breast cancer. -
News Cobra Biologics Receives the Queen’s Award for Enterprise in International Trade
The UK’s most prestigious business award granted in recognition of outstanding achievement in the export of services for gene and immunotherapy products. Cobra Biologics ("Cobra") today announced that it has been awarded the Queen’s ... -
News Shire to sell its oncology business to Servier for $2.4 billion
Sale of oncology business unlocks embedded value within Shire’s portfolio and sharpens focus on core areas reinforcing the company's leadership in rare diseases. -
News Contained Active Freeze Drying could revolutionise pharmaceutical materials drying
A new method of lyophilisation or freeze drying that uses a single, sterilisable, closed vessel to deliver lump free, fine powder product has the capability to revolutionise the production of bulk pharmaceutical powders. With the benefits of improve... -
News Systech International Expands Brand Protection, Track and Trace Solutions to Meet Global Demand and Opens New Training Center Facility
Posted February 22, 2018 in Announcements
Princeton, NJ – (February 22, 2018) Systech International, a global technology leader in brand protection, authentication, and product safety today announced enhanced functiona... -
News Systech Europe™ Announces Rapid Expansion of Customer Base and Pharmaceutical Serialisation Partners Ready to Meet the FMD Regulation
Posted January 12, 2018 in Announcements
Brussels, Belgium – (January 12, 2018) Systech International, the global technology leader in serialisation, track and trace, and brand protection grew its European presence in ... -
News Systech International and NKP Pharma Expand Presence in India to Deliver Pharmaceutical Serialization and Traceability Solutions
Posted November 22, 2017 in Announcements
Princeton, NJ – (November 22, 2017) Systech International, a global technology leader in serialization, product safety, and consumer and brand protection, and NKP Pharma, ... -
News Systech International and Toppan Printing Co., Ltd. Partner to Deliver Brand Protection Solutions through Systech UniSecure™
Posted October 9, 2017 in Announcements
Princeton, NJ – (October 9, 2017) Systech International, a global technology leader in product safety, and consumer and brand protection, and Toppan, a leading integrated pr... -
News Cambrex invests in new continuous flow technology
Investment will focus on the rapid and successful development of processes to supply clinical and commercial demand for chemical syntheses. -
News Pfizer CentreOne launches Enviero green-chemistry progesterone API
Carbon footprint reduced by more than 70%. -
News Catalent completes $5.5 million expansion of clinical storage at Philadelphia facility
Expansion meets the demand for additional capacity to serve large-scale clinical studies and provides the space for future growth. -
News Omega-3s from fish oil supplements no better than placebo for dry eye
NIH-funded study finds omega-3 fails to yield beneficial results in the clinic. -
News Sanofi to invest €350 million in Canadian vaccine facility
New Toronto facility is one of the largest-ever investments by Sanofi in a single building. -
News TraceLink unveils EU FMD Express
Purpose-built compliance solution for smaller pharmaceutical companies. -
News World’s largest dedicated cell and-gene-therapy manufacturing facility opens
First-of-its-kind, state-of-the-art manufacturing facility with capacity to produce treatment for thousands of patients suffering from rare genetic disorders or life-threatening diseases. -
News Bosch presents new processing system for injection solutions
SVP250 LF processing system minimizes product loss thanks to conical vessel shape. -
News Amgen to build first-of-its-kind biomanufacturing plant in the US
Revolutionary, innovative plant offers greater flexibility, speed and efficiency. -
News CPHI South East Asia strengthens ASEAN integration by opening Thailand edition in 2019
The event will annually switch between Indonesia and Thailand to cater to attendees that were previously unable to participate. -
News First GMP-compliant hot melt device for pharma applications
The IHD series has been specially designed for coating and granulating pharmaceutical products with hot greases and waxes. -
News New solutions from Bosch Packaging Technology
Pharma Liquid World shows the connection of a preparation system and filling machine, as well as robotic technology in aseptic filling. -
News PAQXOS – Intuitive and Versatile Control and Evaluation Platform
With the new, intuitively operable control and evaluation software PAQXOS, Sympatec supports first time users as well as experts during particle characterisation. A newly developed, freely adaptable user interface, fully automated system detection, int... -
News BMS awarded 2018 ENERGY STAR Partner of the Year Sustained Excellence Award
The company’s environmental efforts honored for the fourth consecutive year. -
News JXTGE to sell Irvine Scientific to FujiFilm
With the acquisition of Irvine Scientific, FujiFilm will have a full product line supporting customers supplying antibody drugs to assisted reproductive technologies and cell therapy. -
News Servier CDMO Wins 11 CMO Leadership Awards
Servier CDMO is proud to announce we have been recognized across 11 categories in the 2018 CMO Leadership Awards, meeting or exceeding customer expectations in the core categories of Capabilities, Compatibility, Expertise, Quality, Reliability and Serv... -
News BioDuro Collaboration with Pfizer Inc. Leads to Creation of a Shelf-Stable Fluorosulfation Reagent
BioDuro announces creation of AISF ([4-(Acetylamino)phenyl]-ImidodiSulfuryl diFluoride), a convenient, shelf-stable, crystalline reagent for the synthesis of fluorosulfates and sulfamoyl fluorides, in a research collaboration with Pfizer.
-
News Niche CDMO adds spray drying for highly potent drugs
The investment makes Idifarma one of only three companies in the world offering this type of contract development and manufacturing spray drying service. -
News Ardena acqusition adds API and nano medicines expertise
Customers stand to benefit from broader services covering their API, drug product, regulatory CMC and bioanalysis requirements. -
News Recipharm completes blow-fill-seal expansion at Kaysersberg facility
Expansion increases the facility’s manufacturing capacity by 200 million unidoses/year. -
News SEA Vision invests €400K in new serialisation facility at its Tigery facility
Customers to benefit from off-site training and solution testing without the downtime in the manufacturing and packaging processes. -
News bioLIVE to introduce global biopharma country ranking
Global analysis will rank each major biomanufacturing country’s market growth potential, innovation and competitiveness. -
News Top marks for Cherwell’s Redipor prepared microbial media products
Extensive range of ready-to-use culture media available in bottles and plates. -
News Hovione expansion provides integrated full-service solution
Additional capacity expansion across several of the company's sites also strengthens the company's global market leadership in spray drying. -
News Pricing system reform and generics trend apparent at CPHI Japan 2018
Bio and generic demand reflective of wider trends in the market – with overseas investments now prospering. -
News SSB launches new ambr 250 high throughput bioreactor system for perfusion culture
Unique, single-use perfusion system offers a fast-track to intensified cell culture process development.
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance


































.png)









.jpg)






































.jpg)