Top Stories News
Top Stories news
-
News Biologics manufacturing and diagnostics need resiliency, says Scott Gottlieb
Former FDA Commissioner lays out roadmap for building out excess capacity to deal with pandemic situations -
News CPHI Festival of Pharma Podcast - Day 4
The CPHI Festival of Pharma is well underway! Tune into this podcast in which Informa Markets Head of Pharma Content, Tara Dougal, and Pharma Editor, Gareth Carpenter review some of the highlights of the first few days as well as a preview of what's co... -
News What do Pharmapack Awards winners think about patient-centricity?
Pharmapack Awards winners and industry leaders will meet up in a live webinar at CPHI Festival of Pharma to discuss the evolution of next-generation products in pharma packaging, administration & drug delivery devices. -
News Oral Solid Dose Regulatory Hurdles and How to Approach Them
In your quest to meet the regulatory requirements for your oral solids project, you may encounter significant hurdles. Anticipating these early on and thoroughly planning your approach can help. Experts in the Pfizer CentreOne global network, Gillian M... -
News Supporting global industrial scale-up at a pandemic speed with Type 1 pharma glass containers and timely solutions
In a compressed timeframe, producing large quantities of pharmaceutical glass containers to deliver the coronavirus vaccine around the world is a critical factor for a successful global response. Type 1 glass is a proven and widely available solution f... -
News Catalent expands manufacturing support for COVID-19 vaccine AZD1222
The company will prepare the Harmans facility to enable multiple production trains to run in parallel to produce the vaccine candidate drug substance commencing later this year. -
News CPHI Podcast Series: Pharma Action Against COVID-19
Our first in a series of monthly podcasts looks back at the key points covered in five webinars we hosted in May showcasing the pharma industry's response to the COVID-19 pandemic -
News CPHI Podcast Series: The Impact of COVID-19 on API Trading and Procurement
In this podcast, Global Pharma Insights speaks to Stefan Schmidinger, Partner at Kemiex AG on how trading and procurement of active pharmaceutical ingredients has been affected by the global coronavirus pandemic -
News iNova Pharmaceuticals announces the launch of iPitch 2020
Following on from the success of the inaugural iPitch launch in 2019, iNova Pharmaceuticals is delighted to announce the launch of a new round of iPitch challenges for 2020. -
News Pharma packaging companies commit to supply for COVID-19 fight
Stevanato Group, SCHOTT, and Gerresheimer confirm their readiness to support future COVID-19 vaccine with pharmaceutical containers. -
News Emergent bags $628 million "landmark" CDMO deal to produce COVID-19 vaccine candidates
The company will provide CDMO capabilities, capacities, and expertise to support the US government’s Warp Speed Program to pave the way for innovators to advance COVID-19 programs. -
News China focused on meeting international API demand amid pandemic, say officials
Our content has now moved to our new Global Pharma Insights website, providing the very latest news and information covering all aspects of the pharmaceutical supply chain. Please click the link below to access this article. -
News CDMO Suven’s finished drug exports halted due to Indian COVID-19 lockdown
Indian contract development and manufacturing organisation, Suven Pharmaceuticals, has warned it cannot export finished products due to a shortage of raw materials following the country’s recent lockdown to fight the COVID-19 pandemic. -
News 2020 - a year for 'big answers' rather than 'big data'
Zenith Technologies CEO, Joe Haugh, takes us through how the business has evolved this year, to better meet the needs of its customers, as well as the trends they see shaping the industry through 2020 and beyond. -
News Inovio and Beijing Advaccine to advance vaccine against Coronavirus
Agreement will facilitate clinical trial translations in China. -
News AstraZeneca embarks on $1bn zero carbon strategy
The programme will include the launch of next-generation respiratory inhalers, which will use near-zero Global Warming Potential propellants. -
News Diabetes diagnosis - now a matter of sweat and tears?
No need to draw blood; researchers develop smart technology to diagnose diabetes and treat diabetic retinopathy. -
News 2019, the year that got serialization done!
The past 12 months have been busy as many companies fought to comply with serialization regulations. -
News Edible barcodes - it's Tru!
Smart tags on pills set to improve supply chain security and patient adherence. -
News US Pharmaceutical industry continues to shine according to CPHI 2019 Annual Report
The US pharmaceutical industry continues to lead its global peers in terms of innovation and competitiveness, while still ranking high in overall growth potential despite its mature status, according to the results of a survey of 350 global pharmaceuti... -
News UK experiences a 'baby boom' of new life sciences ventures
Unprecedented levels of investment cited as a major force driving the expansion. -
News UK’s life sciences sector continues to attract talent despite Brexit fear
The largest area of growth has been within biotechnology companies, where job openings increased by 35%. -
News Winners of the 16th Pharma Awards
Companies and individuals from across the whole pharma supply chain recognised for their excellence and commitment. -
News ‘FDA should withdraw ANDAs’ says expert
CPHI Worldwide's Annual Report suggests repeat offenders of regulatory infringements should be barred from importing into the US. -
News Sanofi opens its first digitally-enabled, continuous manufacturing facility
One of the first digital manufacturing facilities in the world to use continuous, intensified biologics production technology. -
News EC approves its first plant-derived cannabis-based medicine
Epidyolex (cannabidiol) approved for the treatment of seizures in patients with two rare, severe forms of childhood-onset epilepsy. -
News Integrating glucose sensing and insulin delivery technologies
Collaboration aims to provide a connected device experience for millions of people living with diabetes using insulin. -
News Drivers behind Saudi Arabia’s forecast 10.74 $billion 2022 market
Growing population, increase in non-communicable diseases and major investment in new hospitals, clinics and treatments driving growth. -
News China relaxes import regulations to improve access and availability of affordable medicines
The potential for generics to support the healthcare needs of China is significant. -
News Exciting new vaccine targets killer disease TB
Australian researchers produce early-stage vaccine which is inhaled into the lungs. -
News Switzerland overtakes Germany as Europe’s biggest drug delivery innovator
Pipeline of new drugs has helped drive increased innovation in packaging and drug delivery devices. -
News How real-world data can revolutionise drug development
Utilising the current data environment and its data pools for the benefit of advancing research. -
News Colorcon to incorporate TruTag's edible 'optical barcodes' in tablet film coatings
The benefits of this innovation include the ability to instantly and unequivocally authenticate products when suspect events. -
News Mylan and Pfizer create new global pharma company
The transaction will allow the new company to expand the geographic reach of Mylan's existing broad product portfolio and future pipeline. -
News GSK opens new continuous manufacturing facilities in Singapore
Opening of continuous manufacturing facilities and expansion of production building will boost capabilities to accelerate the supply of new breakthrough medicines to patients globally. -
News Catalent extends global commercial spray drying capabilities in Europe
Company's customers to have immediate access to Niro PSD2 and PSD4 spray driers, which are supported by dedicated clean area facilities for both solvent and aqueous processing of potent or non-potent drug formulations. -
News Inaugural Bio Integrates conference highlights industry's inefficiency in developing products
Industry leaders give voice to issues and trends shaping the biotech sector, including the importance of collaboration. -
News Lessons learned from early EU FMD adopters
Data exchange, investment costs, resources and hardware identified as some of the biggest challenges. -
News Brexit's trick or treat on patient safety
Despite a delayed Brexit, unpredictability adds further complications to the EU FMD regulation. -
News Surge of Indian biosimilars market forecast in 2019
India predicted to be one of the world’s ‘fastest growing bio’ hubs in 2019, fuelled by new biosimilars production. -
News RAPS on delayed Brexit
The post-Brexit regulatory environment continues to weigh heavily on the minds of regulatory professionals. -
News Sizeable growth potential for global drug delivery and packaging in 2019
Patient-centricity, eco-friendly and smart packaging trends marked as the biggest growth drivers and innovations in 2019. -
News Injecting innovation into micro-injection molded solutions
End-to-end industrial production of innovative medical technology products. -
News 'Tamper-evident' benefits are evident
2019 will mark Uniplast's debut at this year's edition of CPHI Worlwide in Frankfurt, where it will showcase its expertise in plastic packaging. -
News All Recipharm facilities ready for EU serialisation, regardless of Brexit
The CDMO invested EUR 35 million into its operations and launched a 3-year programme to provide a compliant serialisation solution for its customers. -
News Brexit - the EU FMD's painful problem
Teething problems are no stranger to the implementation of new regulations, but with the EU FMD, Brexit came like a set of wisdom teeth – late, painful and problematic. -
News Pharmapack report predicts diversification of innovation leading to a rise in licensing and partnering
New report highlights Germany, France and Switzerland as tier-one nations for ‘drug delivery innovation’, and warns the challenge will be to scale-up and approve promising prototypes. -
News New API screening program strengthens Particle Sciences' nanomilling offering
Advanced formulation techniques such as nanomilling may provide an excellent route to improved bioavailability and enhanced therapeutic effect. -
News Univercells introduces breakthrough vaccine manufacturing platform
The automated NevoLine bioproduction system that facilitates safer, faster and closed bioprocessing in a much smaller footprint. -
News BMS and Celgene merge to create premier innovative biopharma company
Significantly expands Phase III assets with six expected near-term product launches, representing greater than $15 billion in revenue potential. -
News An increased risk of a hard or ‘no deal Brexit?
A ‘no deal’ Brexit will change and burden how all industries that move materials and goods across borders, including the pharmaceutical and medical device sectors, do business. -
News One month on: medical cannabis is still taboo despite change in law
Restrictive guidelines have led many to buy CBD products online, leading to a boom in production with many products going to market without sufficient quality control. -
News EU FMD - time is running out!
Companies that have left it too late to implement their own solution will have to rely on support from providers, whose resources are already in high demand as the deadline approaches. -
News Proprietary nasal delivery formulation of diazepam reaches NDA
The absolute bioavailability of the Valtoco intranasal formulation was 96% of intravenous diazepam in a Phase I cross-over trial. -
News DSCSA one-month countdown!
With only a month to go, it really is too late to start trying to develop an in-house solution. -
News Global pharma set for strong year
Eight of the top ten pharma nations reporting improving market conditions. -
News Generics companies to move away from "little white pills"
Accepting more complexity and risk is key for those generics companies seeking competitive differentiation by diversifying their product portfolios. -
News CMOs to benefit from double digit approvals for ADCs in the next 3 years
Therapeutic ADC market expected to reach $4 billion by 2023. -
News Movement on the market reflected at CPHI Worldwide 2018
Companies new to CPHI to showcase an exciting range of products and services ranging from electroceuticals to refrigerant alternatives. -
News Moulds & Tooling: A European success story
While not quite an endangered species, the past fifteen years have not been easy ones for many European mould makers. Many, but not all, and IGS GeboJagema is a case in point – a case that shows the difference automation and focus can make. By Karen La... -
News Sanofi to refocus two global business units
The new Primary Care and China & Emerging Markets global business units are expected to launch at the beginning of 2019. -
News Experts warn trade and patent changes could increase healthcare cost by $100bn over next 5 years
China is harmonizing to ICH standards at feverish pace and will force poor quality manufacturers out of market. -
News Exciting skincare to protect against blue light emitted by phones and devices
Novel, dual-acting product revitalizes and protects skin against blue light and environmental aggressors around the clock. -
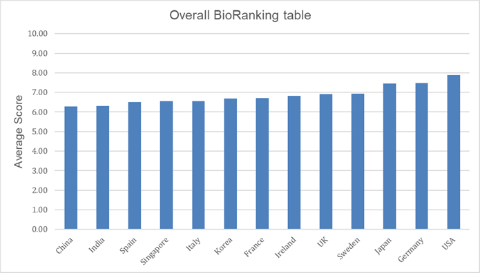
News Bio processing and manufacturing league table ranks US top and China bottom
US, Germany and Japan revealed as tier one nations in all categories with Sweden ranking the best of the rest. -
News Innovation "hindered by regulators"
CPHI Annual Report expert sees CDMOs as potentially a source of process innovation but warns both regulator and license holders need to move past regulatory diktats. -
News The 6-month FMD countdown - are you ready?
With only 6 months left in which to become EU FMD-compliant, how can companies make the best use of their now limited options? -
News Protecting the links between the UK and the EMA
Now less than a year away from when the UK officially leaves the EU, the main goal remains the same - maintaining access to new drugs for patient health. -
News Building contingency plans for an ambiguous post-Brexit market
One company's positive approach to Brexit preparations, despite the lack of definition. -
News Post-Brexit medicines - stuck in the UK?
The EMA needs to provide more action and guidance to help UK pharmaceutical manufacturers, says Thomas Beck, senior vice president, quality management, Recipharm. -
News Pharmacovigilance post-Brexit - simpler than previously thought?
To meet new PV regulations, the industry has to face a "multitude of unknowns and an ever-moving target". -
News South Korea's finished drug market growth driven by generics
FDF launches at CPHI Korea as country set to become regional hub. -
News Bosch to sell its packaging business
Packaging technology is not a core Bosch business. -
News Ingredients to improve bioprocess manufacturing efficiency
Experts warn bio industry needs to do more to create a sustainable pipeline of talent. -
News Internationalisation and regulatory reform driving growth and investment at CPHI China
2018 event prepares for surge in international attendees as MAH nears national rollout. -
News Investing in blistering capabilities to boost flexibility
Postponement printing is presenting interesting benefits for the pharmaceutical supply chain. -
News Serialisation — looking for value-added opportunities
Recipharm's Staffan Widengren, Director Corporate Projects, discusses why CMOs should be focusing on the value-added opportunities presented by serialisation. -
News Omega-3s from fish oil supplements no better than placebo for dry eye
NIH-funded study finds omega-3 fails to yield beneficial results in the clinic. -
News bioLIVE to introduce global biopharma country ranking
Global analysis will rank each major biomanufacturing country’s market growth potential, innovation and competitiveness. -
News Technology and managing risk within the high potency drug sector
Manufacturing high potency drugs - the challenges and innovations to mitigate the risks. -
News Lonza expands encapsulation and HPAPI capabilities in North America
Customers to have the option of one partner from concept to market for their encapsulated drug products -
News Company goes all out to fully support clients
Gaining GLP/GMP accreditation to provide greater assurance of data integrity for clients looking to take their compounds to IND. -
News Acquisition taps into the unknown treasures of the sea
Screening for new marine-derived functional ingredients and bringing new patented products to the market. -
News Patient centricity and innovation a winning formula at packaging awards
Packaging innovation shows a clear focus on patient compliance and ease-of-use. -
News Best practice tips for selecting a prepared media supplier
What does a good supplier look like? Andrew Ramage, Microbiology Product Specialist at Cherwell Laboratories, advises how to take the guesswork out of the search. -
News A powerful new tool for the separation of charged species
A new analytical tool, Electrical Asymmetrical Flow Field-Flow Fractionation (EAF4) is showing much promise in biopharmaceutical and nanoparticle applications. -
News 2017: A year of innovation and advances
A summary and highlights of new drug therapy approvals and other drug therapy advances in 2017 by FDA's CDER. -
News Teva to axe 25% of its total workforce
Total cost base to be reduced by $3 billion by the end of 2019. -
News Major breakthrough for new haemophilia A treatment
Drug trials provide mind-blowing results. -
News Major pharma company to make significant investment in the UK
MSD's announcement to establish UK Discovery Centre in London an 'endorsement' of the government's Industry Strategy. -
News Key trends in formulation development for 2018
Controlled-release technologies will continue to be focal point, whereas techniques to improve solubility are likely to gain popularity. -
News Recipharm to end operations in two facilities in Sweden
Company's decision affects approximately 225 staff, specialised in tablet manufacturing, and in sachet and stick pack filling, primarily for powders and granules. -
News CPHI Worldwide announces the winners for its 14th Pharma awards
19 winners chosen from 200 entries, received from nearly 30 countries worldwide. -
News Will Novo Nordisk's semaglutide be its next blockbuster?
Semaglutide receives positive 16-0 vote in favour of approval from FDA Advisory Committee. -
News Boosting capabilities through collaboration
CDMO, Micro-Sphere partners with German machine manufacturer Harro Höfliger for the latest step in its 21 million CHF (€19 million) investment into its facility in Switzerland. -
News Highly potent APIs - can your CMO handle them?
The importance of ensuring a facility has the right capabilities when selecting a CMO to handle high potency ingredients. -
News FDA warns Pfizer company about cGMP violations associated with the manufacture of EpiPen
Warning letter cites Meridian Medical Technologies' failure to thoroughly investigate multiple serious component and product failures of EpiPen products. -
News Novo Nordisk settles US federal investigation of marketing practices
In connection with the $60 million settlement, the company has also resolved several private whistle-blower cases related to the government's investigation. -
News Mylan finalizes settlement agreement on Medicaid rebate classification for EpiPen Auto-Injector
Mylan will reclassify EpiPen Auto-Injector for purposes of the Medicaid Drug Rebate Program and pay the rebate applicable to innovator products effective as of 1 April 2017. -
News Lonza acquires Micro-Macinazione to create the leader in micronization capacity and capabilities
Bolt-on acquisition adds value to other areas of Lonza’ business, including Health & Nutrition ingredients and excipients. -
News Artificial intelligence to discover drugs in a multi-year multimillion dollar deal
AI thought to have massive potential to reduce the cost associated with the discovery of new drugs. -
News Merck and Pfizer collaborate with Corning to modernize pharmaceutical glass packaging
Collaborations result in development of Corning Valor Glass. -
News GSK delivers double-edged sword
Company announces investments, the sale of several products and a proposal to close a UK manufacturing site. -
News Researchers develop microneedle patch for flu vaccination
The patch can dramatically reduce the cost of vaccination, as self-administration can eliminate the need to have health workers oversee the process. -
News Takeda terminates plans for Vaxem Hib launch in Japan
Discontinuation due to the vaccine supplier’s (GSK) decision to discontinue global production. -
News Croda celebrates EXCiPACT Certification across all leading excipient sites
Status marks the company as the first multi-site excipient supplier to achieve global EXCiPACT accreditation across its production facilities. -
News Chemotherapy as a tablet instead of an intravenous infusion
New production method for solid dispersions of docetaxel and paclitaxel. -
News Landmark outcomes study shows that Repatha decreases LDL-C to unprecedented low levels
Amgen has announced that the 27,564-patient Repatha (evolocumab) cardiovascular outcomes study, FOURIER, established for the first time that maximally reducing low-density lipoprotein cholesterol (LDL-C) levels with Repatha, beyond what is possible wit... -
News Mylan announces global settlement and license Agreements with Genentech and Roche on Herceptin
Settlement provides Mylan with global licenses for its trastuzumab product. -
News Pharma's most innovative companies; the 2017 ranking
Innovation is being driven increasingly by smaller, possibly more agile, companies. -
News Can yeast rise to the challenge of curing cancer?
Modified baker’s yeasts cause an immune response and the production of lymphocyte killers, which detect and eliminate cancerous cells. -
News Molecule shows ability to thwart pathogens’ genetic resistance to antibiotic
The molecule, which is a PPMO, will likely be ready for testing in humans in about 3 years. -
News Selonterra publishes novel proprietary mechanism for APOE4 in Alzheimer’s disease
Therapies targeted at this novel mechanism have the potential for disease-modification because they will address the genetic root cause of AD. -
News Ariad to be acquired by Takeda for $5.2 billion
Ariad stockholders to receive $24.00 per share in cash. -
News Mylan launches the first generic for EpiPen Auto-Injector as an authorized generic
Authorized generic offered at more than 50% below wholesale acquisition cost of EpiPen auto-injector. -
News Lonza to acquire Capsugel
Acquisition will make Lonza a fully integrated solutions provider in oral delivery technologies and active ingredients to the consumer healthcare and nutrition markets. -
News BMS strengthens capabilities with evolution of its US geographic footprint
Company will also close its Hopewell, New Jersey site and will not renew its lease at the Lake Union Steam Plant site in Seattle, Washington. -
News Pfizer refutes the findings set out in the CMA decision
Pharma giant says it complied with established competition law. -
News Hopes dashed for new Alzheimer drug
Lilly's solanezumab fails to meet primary endpoint of Phase III study. -
News Pfizer discontinues global development of bococizumab, its investigational PCSK9 inhibitor
Bococizumab deemed unlikely to provide value to patients, physicians, or shareholders. -
News Fighting cancer with the power of immunity
New treatment elicits two-pronged immune response that destroys tumors in mice.
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance





.jpg)



.jpg)
.jpg)


.jpg)
.jpg)
.jpg)


.jpg)

.jpg)
















.png)


.jpg)
%20(2).jpg)

.jpg)





































.png)
_Logo.jpg)
























