CPHI Online is the largest global marketplace in the pharma ingredients industry
-
Products551,744
-
Companies7,781
-
Articles11,636
-
Events8
-
Webinars342
Formulation development
Formulation development Companies (69)
Formulation development News
-
News CPHI Barcelona 2023 Pharma Awards: and the winners are...
The much anticipated Pharma Awards 2023 were held at CPHI Barcelona this week at the Fira Barcelona.25 Oct 2023 -
News On track at CPHI Barcelona - The Track Sponsor interview: Lonza
In our packed out content sessions at CPHI Barcelona this year we focus on some of the hottest topics coming up in the pharma industry, with each track sponsored by a leading expert in the field.23 Oct 2023 -
News Choosing the Right CDMO Partner: A Comprehensive Guide
Finding the right partner for the development and manufacturing of your pharmaceutical or biopharmaceutical products is paramount. This is where Contract Development and Manufacturing Organizations (CDMOs) step in, offering their expertise and infrastr...28 Aug 2023 -
News CordenPharma Increases xRNA-based Capabilities at its Caponago Injectable Facility
CordenPharma, a full-service CDMO of APIs, Excipients, Drug Products & Packaging services, has increased its xRNA capabilities at its sterile injectable facility in Caponago, Italy.1 Jun 2022 -
News Catalent fills gap in the market with new development offering, Xpress Pharmaceutics
Xpress Pharmaceutics will facilitate adaptive clinical trials and reduce the time taken to complete FIH studies26 Jan 2022 -
News CPHI Podcast Series: Orally Dissolving Tablets
In this month’s CPHI podcast, sponsored by Galien Pharma, we focus on the technology behind orally dissolving tablets.4 Jan 2022 -
News Adare Pharma acquires oral formulations specialist Frontida BioPharm
The combined company intends to lead in the space of oral formulations, solving customers' complex formulation challenges3 Dec 2021 -
News Lonza signs five-year collaboration deal with VC firm Bioqube to speed up portfolio companies’ development and manufacturing
The offering will accelerate timelines for the development and manufacturing of molecules and disruptive technologies30 Nov 2021 -
News Lonza Switzerland site to undergo expansion of microbial development capabilities
The expansion includes the installation of a pilot suite with a 50-L fermenter and automation upgrades to accelerate clinical and commercial projects17 Nov 2021 -
News Hoth Therapeutics and WuXi strike API and drug product deal for cancer therapeutic
WuXi will use its new modality platform to provide an end-to-end solution for oligonucleotide peptide12 Nov 2021 -
News Indian pharma industry on cusp of growth spurt, CPHI Worldwide audience told
Country expected to grow threefold over the next decade due to increased focus on complex generics, biosimilars and specialty products26 Oct 2021 -
News Formulated Solutions completes investment and expansion project
The CDMO significantly increases its footprint, capacity and capabilities to meet current and future demand6 Sep 2021 -
News Skyepharma selects Veratrak platform to ensure secure data storing and sharing
Using Veratrak's platform, the CDMO will have full oversight of data, including an audit trail of data and document processes20 Jul 2021 -
News Curia to buy fellow CDMO Integrity Bio
Acquisition will add West Coast coverage to the CDMO's East Coast and European capabilities and enhance its biologics formulation development and fill-finish network14 Jul 2021 -
News CPHI Webinar: Trends and Advances in Patient Centric Drug Development - Watch On Demand
Catch up on the latest trends and developments in patient centric drug development23 Jun 2021 -
News Coriolis Pharma expands facilities for ATMP development
The expansion close to company's Martinsried headquarters includes a lyophilisation development centre for advanced therapy medicinal products23 Jun 2021 -
News The Top Ten CPHI Discover Company Showcases
CPHI Discover (May 17-28) gave Pharma companies, suppliers and service providers the opportunity to keep potential buyers and partners abreast of the latest news, product information and market trends in the form of free-to-access, downloadable content...3 Jun 2021 -
News MedPharm expands topical and transdermal delivery services
A new location in Raleigh-Durham, North Carolina, close to the CDMO's Center of Excellence, will double its current footprint2 Jun 2021 -
News UPDATED: The CPHI Discover Blog
Bookmark this page for regular updates on the rich variety of content coming your way via CPHI Discover, pharma’s largest ever virtual gathering, over the course of our three-day agenda20 May 2021 -
News CrystecPharma opens R&D facility in Haimen City, China
Facility will support UK-based crystal and particle engineering specialist's existing Chinese subsidiary to expand product development services31 Mar 2021
Formulation development Products (230)
-
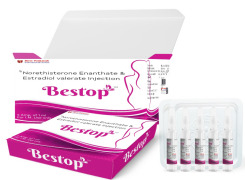
Product Norethisterone Enanthate and Estradiol Valerate injection-Bestop
Norethisterone Enanthate and Estradiol Valerate injection packed in ampoule for Intramuscular injection.
Each ml of injection caontains Norethisterone Enanthate 50mg and Estradiol Valerate 5 mg.This medication is used to prevent pregnancy in women.

-
Product Formulation Development
Within early development, there are a variety of different challenges that hinder a molecule’s ability to progress to the next phase such as complex formulation.
The complexity of formulation development has been on the rise, which is driven by a variety of different factors including:
...
-
Product Biologic drug substance CDMO services
From pre-clinical development to commercial supply, Patheon by Thermo Fisher Scientific is an industry leader in the development and manufacture of mammalian cell culture drug substances. Patheon offers biotech and pharmaceutical companies the ability to pursue opportunities around the globe with a fully i...
-
Product SIMETHICONE RANGE (Gastro metabolism - Dyspepsia - Aerophagy- Infant colic)
Simethicone is a stable mixture of silicone polymer and silicone dioxide containing anti-foaming and intestinal gas reducing properties. It is indicated in the treatment of dyspeptic disorders and gastroenteric meteorism. Simethicone decreases the surface tension of mucus and of gas bubbles ...
-
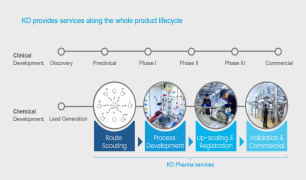
Product CONTRACT MANUFACTURING - SMALL MOLECULES
KD Pharma’s professional expertise, state-of-the-art assets and proven track record in regulatory compliance allow us to become your preferred CDMO solutions partner.
Your Agile Experts
• Responsive to our customers’ needs in a more dynamic customer-centric way • Adaptive to the most diverse re...
-
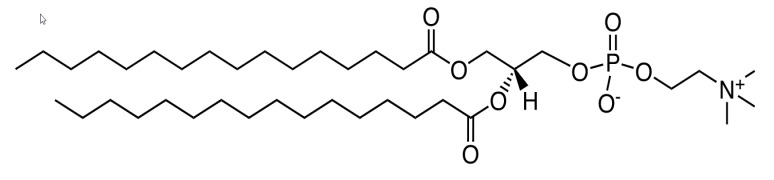
Product CordenPharma Derivatized Phospholipids - DPPC & More
CordenPharma’s expert lipid manufacturers have mastered the chemical total synthesis of complex phospholipid derivatives from multi-gram to multi-kilogram scale and are renowned for their contract lipid and phospholipid molecule manufacturing expertise. Our large-scale proprietary cGMP manufacturing proces...
-
Product Inhalation Drug Product Development Services
Services for the development of inhalation drugs: Our team have over 30 years of experience supporting our clients' product development for orally inhaled or nasal drug products (OINDP). This includes formulation, stability, testing, product performance testing, in vitro bioequivalence studies, CMC...
-
Product Ophthalmic CDMO Services
Salvat offers the best services and solutions, drawing on a long history of expertise in developing and manufacturing successful medications.
Tailored solutions to customer needs through our Ophthalmic platform: • • Customized solutions using Salvat's technology: suspensions, solutions, micellar,...
-
Product UniLayer - 2mg Loperamide
This product is suitable for children as a delivery system.
Must be taken with water or beverage of choice.
We are looking to partners on a CDMO bases.

-
Product Spray Drying
We have been successfully developing spray dried formulations for our customers for more than twenty years. Our experience spans a vast range of therapeutics from small molecule API’s to even the most sensitive and complex biologicals.
-
Product ENNA MOISTURIZING GEL VAGINAL DRYNESS
ENNA MOISTURIZING GEL AGAINST VAGINAL DRYNESS. MEDICAL DEVICE. VEGAN. CLINICALLY AND GYNECOLOGICALLY TESTED
-
Product Succinyl Gelatin Injection
Succinyl Gelatin Injection is a plasma volume substitute. This means, it replaces fluid lost from the circulation. It is used to replace blood and body fluid, which have been lost as a result of, for example, an operation, an accident or a burn. It can be used instead of, or as well as, a blood transfusion...
-
Product Formulation Development
Singota solutions offers wide range of services which includes analytical and formulation development. We create a robust method for analysis of product, whether starting from scratch or optimizing a method, and can help create a set of solutions that are customized to you, while supporting that metho...
-
Product Pharmaceutical development (CDMO) & Exclusive portfolio
From research, pre-formulation, and formulation development, up to commercial manufacturing (including HPAPI).
Bluepharma offers an integrated drug development service (CDMO) from research, pre-formulation, and formulation development, up to commercial manufacturing ...
-
Product BerryShield UTI gummy
A herbal blend of cranberry and Hibiscus in a delicois gummy: Ingredients with high consumer awareness for urinary tract health. Pectin-based, with no-added sugar.
-
Product HyperStart C2C Smart Formulation Hub
Digital self-serve platform to boost R&D productivity through interactive formulation development.
-
Product Fourier PAT
We celebrate the arrival of our synTQ by Optimal NMR adapter – opening the door to a new world of PAT for the Bruker Fourier 80. This brings a wealth of structural information and direct quantification to online chemical and bioprocess monitoring, resulting in further reductions in risk and cost.
-
Product Inhalation Test Services
We are the world’s leading provider of orally inhaled and nasal drug product design and development services. We enable the seamless translation of pre-clinical development through to clinical manufacture of OINDPs. We do this through our unique processing technologies and formulation development tools....
-
Product OTC and MD dossiers
A list of regulatory OTC dossiers compliant with European regulations and in CTD structure (NEES-eCTD) is available. These dossiers are ready to be audited by clients and to be submitted for registration to Health Authorities. We provide support during the registration process until the dossiers are author...
-
Product VELOXTAR™ technology
VELOXTAR™ is a technology that produces a very thin orally disintegrating tablet (ODT) like a coin. The distinctive tablet shape and unique formulation design allow the ODTs to disintegrate in 4-6 seconds. VELOXTAR™ technology significantly reduces the disintegration time, while still providing practical t...
-
Product Formulation Development
The NUVISAN formulation group up has a proven heritage of effective product development with team members being responsible for the creation of topical formulas that supported several commercially successful products. These successes were underpinned by our focus on innovation and patient and health care p...
-
Product Hot Melt Extrusion
To successfully develop and manufacture your polymer-based delivery systems, like implants and intra-vaginal rings, we have advanced extrusion capabilities in Malmö (Sweden) and Putnam (US Connecticut). The US site is also a center of expertise for Hot Melt Extrusion (HME).
HME improves the bioa...
-
Product Formulation Development
KBI’s approach to formulation development is based on the strategic pairing of two complementary scientific disciplines:
• Establishing a comprehensive understanding of the thermal, physical, chemical, and conformational stability. • Employing statistical design-of-experiment (DOE) to evaluate main ef...
-
Product Thermal characterization of the product
Having information on the thermodynamic behaviour of our formula is critical to identify the critical product temperatures. At Comser, we have the following technology to characterize the product:
- Freeze Drying Microscope (FDM)
- Differential Scanning Calorimetry (DSC)
And with such,...
-
Product OTF - Oral Thin Films
Oral thin films are loaded with active substances. The Thin films are taken orally and dissolve immediately in the mouth or are applied to the mucosa. For transmucosal films, the active substance enters the bloodstream directly via the oral mucosa, without having to first pass through the gastrointestinal ...
-
Product Parvulet® Technology - Patient Centric Dosing Solution
Adare has recently launched a new patient centric dosage solution, the Parvulet TM Technology. The Parvulet TM Technology enables a solid powder or tablet to convert to a semi-solid in the presence of water within thirty seconds. The final dosage is easily administered, as a soft food like texture, ide...
-
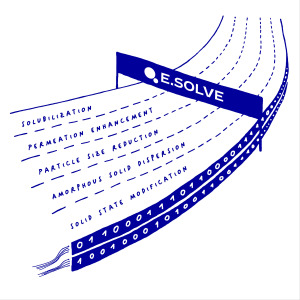
Product E.SOLVE | SOLVING BIOAVAILABILITY CHALLENGES
E.SOLVE accelerates your journey to the clinic supporting as early as possible your formulation strategy from discovery to market approval.
· When you deal with a challenging API, partner with Evotec’s screening experts to guide your process through every challenge along the drug discovery and devel...
-
Product Continuous Manufacturing of Formulations in Minutes Instead of Hours
Microinnova Engineering GmbH has successfully commissioned two continuous formulation production plants for a European client. The design of the fully automated plant is very compact, which makes it possible to easily transport & install it at a different manufacturing site on the other side of the wor...
-
Product Spray-drying/spray-congealing
Spray Drying is an instant and continuous solvent evaporation process of a liquid feed, atomised in a heated gas, generating a final dried solidified particle.
The heated drying gas moves in co-flow with the atomised feed in the process chamber where the gas transfers its heat energy to the ...
-
Product R&D Laboratory of medical devices
Over 400 developed recipes,
including over 50 medical devices in various classes and indications,
in 20 pharmaceutical forms -
everything starts in the Gofarm's R&D Laboratory.
In Gofarm's R&D Laboratory our and our clients' ideas take real shapes...
-
Product ADAPTEK® TECHNOLOGY
ADAPTEK® TECHNOLOGY is the general name for our 4 international patents and expertise in nanotechnology and controlled release systems.This is a transversal technology that allows to develop customized biopolymeric nanohydrogels, allowing the load with different API (drugs, vitamins, growth factors, etc.)....
-
Product Formulation Development
Recipharm offer formulation development services for all dosage forms. We develop everything from simple formulations for early studies to more complex formulations suited for commercialisation.
-
Product Parenteral drug delivery solutions
Evonik is one of the world’s leading CDMOs for parenteral drug delivery. For complex parenteral drug products designed for systemic, targeted or localized delivery, we are uniquely positioned to serve as a global development partner and solutions provider. Our parenteral drug delivery portfolio includes th...
-
Product Contract Development Services
Famar R&D is offering a complete set of Contract Development services.
• Formulation and process development for Rx, Gx, OTC, Cosmetics (Solids, Semi-solids /liquids dosage forms, Sterile liquids & Lyophilized powders) and Medical Devices • Analytical methods development and validation&nbs...
-
Product Pharmaceutical R&D Services
Having decades of hands-on experience on a wide range of (complex) technologies and a many different types of products, our Pharmaceutical experts are able to fully execute and/or support the development of any finished dosage form, formula or process, as well as the transfer and scale-up of a deve...
-
Product Formulation Development
Quotient Sciences has almost 30 years of experience developing a breadth of pharmaceutical formulations across a range of indications. Our innovative approach to formulation development integrates drug product development with clinical evaluation, combined with our experience with over 1,000 molecules at a...
-
Product Contract Development and Manufacturing
We are a Pharmaceutical contract development and manufacturing company authorised for the preparation of medicinal products and medical devices in liquid, suspension, gel and cream form, lyophilisates and ointments. Our services include formula development, pilot studies in the laborato...
-
Product Formulation Development
MedPharm has more than 20 years’ experience developing topical and transdermal formulations. We can begin as early as API screening and support pre-formulation activities, lead formulation selection, formulation optimization and reverse engineering. We have had a role in taking over 55 products to mark...
-
Product Early stage formulation Development & Process Design
We have extensive experience in solving complex formulation challenges. Our experts can apply our extensive range of proprietary technologies for immediate (IR) and modified (MR) / sustained, slow (SR) / controlled (CR) / extended (XR, ER) / pulsed / timed release as well as enteric and gastro reten...
-
Product Inhaled Formulation Development
With expertise in powder and liquid formulations for small molecules and biologics, as well as a broad portfolio of proprietary device and formulation technologies, we can help you overcome the challenges of inhaled formulation development.
Dry Powder Formulation
Advanced po...
-
Product Formulation development
Alcami’s formulation development team proactively guides your development program every step of the way – from Compound to Clinic. Our team is adept at solving challenging formulations for new chemical entities (NCEs) and developing high-performance dose forms to meet today’s consumer needs. We offer...
-
Product Formulation and Analytical Solutions
GVK BIO offers a range of Formulation R&D solutions that include pre-formulation studies, formulation development, analytical R&D, reformulation and stability studies. We can also support clinical supplies and manufacturing of exhibit batches in collaboration with our partners and offer standalone ...
-
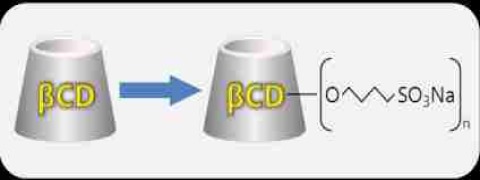
Product Dexolve
Dexolve is the first generic USP and EP-conform Betadex Sulfobutyl Ether Sodium for:• solubility enhancement (10 to 100,000 fold) • Improvement of chemical stability • Increased bioavailability, facilitated delivery • Reduced aggregation • Moderate irritationor reduced side-effects • Maximiz...
-
Product Product Development
Our product development team is highly experienced in the realization of product ideas. Finding the optimal formulation for medicinal products, medical devices and food supplements with highest quality standards is our primary aim. We can help you in pursuing your idea. Contact us now to learn more abo...
-
Product Tablets, bolus and effervescent tablets
Dry forms : tablets, effervescent tables, bolus, powders ... filled in specific packaging according to species, product, dosage, use : plastic bottle, aluminium pouch
-
Product ITRACONAZOLE PELLETS 22% W/W:
ITRACONAZOLE PELLETS 22% W/W:
Specifications: White to off white spherical pellets,
Pellets Size: ASTM16#20:
Release specification: As per latest USP std.
Fill Weight:
For 100mg capsule: 455mg pellets filled in SIZE 0 capsules. 1 kg of Pellets can ...
-
Product DELOS FORMULATION TECHNOLOGY
Development and Manufacturing of nanomedicines and tailored drug delivery systems using DELOS patented nanoparticles platform. DELOS is a formulation technology which is enabling:
ENHANCED DRUG PRODUCTS:
• Reformulate drug candidates, small molecules or biologics, with poor...
-
Product DILTIAZEM HCl S.R. CAPSULES 200 mg - 300 mg - (New Product)
Valpharma provides a wide range of products which inlcudes DILTIAZEM HCl S.R. CAPSULES 200 mg - 300 mg for the treatment of hypertension, angina pectoris and some types of arrhythmia.Diltiazem is a nondihydropyridines (non-DHP) calcium channel blocker used in the therapy and prophylaxis of coronary ins...
-
Product Contract Formulation Development & Analytical Development (CDMO)
Contract Research and Development (Fee for Service)
Solids-
• Tablet, Capsule, PFOS, Granules, Sachets, Pellets • Oncology Potent and Veterinary products • MR,ER,SR,DR,GR,PR Dual Release • Mini-Tablet in Capsule, MUPS, Hot Melt Extrusion Injectables • Liquid, Lyoph...
-
Product Formulation Development
Avéma has an extensive library of formulations and more than 40 years of Rx formulation development experience.
-
Product AUTOMATIC TABLET COATING - PROCOTA:
Salient Features: -
⇛ Design is cGMP – Current Good Manufacturing Practices compliance.
⇛ All contact parts AISI 316 & non-contact parts AISI 304.
⇛ Fully automatic film Coating by PLC Controls with HM...
-
Product Drug Product Pre-Formulation and Formulation
CoreRx provides comprehensive drug product pre-formulation, formulation, analytical, and GMP manufacturing and packaging solutions, enabling our partners to meet their drug program goals. CoreRx delivers these solutions from it’s development and manufacturing campus in Clearwater, Florida and it’s...
-
Product Contract Manufacturing & Private Label
From concept to logistics, our product development capability and global market knowledge gives us a long-term sustainable advantage. Additionally, our Bangalore Whitefield facilities are EU CGMP Certified with 3 in-house laboratories.
Equipped with the latest technology, all our products ...
-
Product Formulation, Process & Device Department
• Compatibility studies and preformulation • Formulation and lab-scale batches • Definition and supervision of in-vivo studies • Non-GMP and GMP activities • Med-Tech workshop for device prototyping
-
Product August Bioservices - End to End (Preclinical to Clinical to Commercial), US-based CDMO Capabilities
This is an overview capabilities brochure of the services and expertise August Bioservices offers to biotech and pharma companies of all sizes. As a growing, US-based provider of expert CDMO services, August is rapidly becoming a preferred partner for clients seeking deep drug development, formulation, sca...
-
Product CMO Services
CMO services could be offered for Oral Solid forms - especially for Oncology products - in PharOS manufacturing plant in Malta.
-
Product Research & Development and Regulatory Affairs
In order to carry out its activities of R&D, prototyping and pilot batches preparation, Enable Innovations avails itself of a brand new production unit equipped with fully automated filling and packaging lines and laboratories complying with the most restrictive applicable regulations for medical devic...
-
Product Pharmaceutical Development
Expert formulation and process development for sterile injectable drug products.
We take a Quality by Design (QbD) approach in accordance with ICH Q8 to ensure critical processing parameters (CPPs) are verified against critical quality attributes (CQAs)
· ...
-
Product LOZENGES
Lozenges offer a variety of options for the manufacturing of chemical and natural-based ingredients.
Our focus: APIs with local effect as well as APIs that are absorbed orally.
Fast and flexible: We're able to process a variety of substances and can quickly switch between differen...
-
Product ALCORUB (Alcoholic Rub-in-Hand Disinfectant with Emollient & Moisturizer)
ALCORUB
Each 100 gm Contains :
2-Propanol I.P. ...
-
Product DuPont Nutrition & Health
• AVICEL PH • AC-DI-SOL • AVICEL RC/CL • AVICEL SMCC • AVICEL DG • AVICEL HFE 102 • AVICEL CE 15 • AQUACOAT • AQUATERIC N100 • ALUBRA • PROTACID/ PROTANAL • GELCARIN / VISCARIN • SEAGEL
-
Product Research and Development of new products at SVP High-tech R&D centre
SVP is willing to cooperate with partners in Research and Development of new products at SVP High-tech R&D centre, including high-tech products, special formulations, liquid products, biopharmaceutical products, oncology products and technology transfer support. For more detail, please contact us.
-
Product New Product Development
Pellets Pharma Limited provides various pharmaceutical products which includes new development products including Dimethyl Fumarate, Phentermine +Topiramate, Aspirin+Dipyridamole, Levomilnacipran, Memantine+Donepezil, Nimesulide, Fenofibrate (Nano) 20% DC granules, Fenofibrate...
-
Product Abamectin 1.0% EC
North china pharmaceutical group corporation offers a wide range of finish pharmaceutical which includes Abamectin 1.0% EC . It belongs to pesticide formulation. Major control objects : rice leaf roller, chilo suppressalis walker, scirpophaga incertulas, cotton bollworm, kanazawa spider mites, plutel...
-
Product Clinical supplies
Syngene International Ltd. provides wide range of services which includes clinical supplies. It belongs to formulation development services category. It includes scale range - 0.1 kg to 50 kg, packaging - bottles and blister packing. Contact us for more information.
-
Product Deflazacort
CTD-DMF
Anti-inflammatory & immunosuppressant. USDFA granted fast track to use as a potential treatment for Duchene Muscular Dystrophy in US
-
Product Methyl Prednisolone Aceponate
CTD-DMF
Atopic Dermatitis (Endogenous Eczema, Neurodermatitis), Contact Eczema, Degenerative, Dryshidrotic, Vulgar Eczema, Eczema in Children, Psoriasis
-
Product Sterile drug product CDMO services
Thermo Fisher Scientific's flexible aseptic manufacturing and sterile fill finish solutions for your molecule’s unique needs and challenges will enable success in early development, late-phase, and commercial manufacturing.
Thermo Fisher offers extensive sterile product development and commercial ...
-
Product Core Technologies and Services
• API / GMP Manufacturing • Rapid Process Development, Flawless Upscaling, and Economy of Scale-Production • Simulated-Moving Bed (SMB) Chromatography • Heterocyclic, Hazardous and Malodorous Chemistries • Organometallic and Cryogenic Chemistry • Transition-Metal Catalysis • High-Pressur...
-
Product Formulation Development including Inhaled Products
Inhaled products and formulation development: The development of drugs using experimental design approaches with integrated stability testing and storage is available from us. Our formulation specialists can provide a wide range of formulations in a timely and cost-effective manner in order to identify t...
-
Product Fine Chemicals
SPECIAL TECHNOLOGIES AND REACTIONS
Special Technologies
• High-pressure reactions up to 64 bar: H2, CO, NH3, Amines, CO2 • Cryogenic: -80°C • Supercritical Fluid Chromatography (SFC), Simulated Moving Bed Chromatography (SMB) • Ultra low vacuum distillation • ...
-
Product Clinical Trial Supplies Manufacturing Services
Services for manufacturing clinical trial supplies: To meet your supply needs for investigational medicinal products (IMP) or investigational new drugs (IND) for clinical trials around the world, we provide GMP clinical trial materials manufacturing services. Integrated with raw material testing, ...
-
Product UniLayer - 200mg Ibuprofen
This product is suitable for children as a delivery system.
Must be taken with water or beverage of choice.
We are looking for partner on a CDMO bases.
-
Product UniLayer - 5mg Montelukast Sodium BP
This product is suitable for children and adults, as a delivery system when taking this medication.
Must be taken with water or beverage of choice.
We are looking for partner on a CDMO bases.
-
Product UniLayer - 10mg Cetirizine Hydrochloride
This product is suitable for children and adults, as a delivery system when taking this medication.
Must be taken with water or beverage of choice.
We are looking for partner on a CDMO bases.
-
Product UniLayer - 200mg Paracetamol
This product is suitable for children and adults, as a delivery system when taking this medication.
Must be taken with water or beverage of choice.
We are looking for partner on a CDMO bases.
-
Product Dopamine Hydrochloride Injection
Dopamine Hydrochloride Injection is indicated for the correction of hemodynamic imbalances present in the shock syndrome due to myocardial infarction, trauma, endotoxic septicemia, cardiac operation, renal failure, and chronic cardiac decompensation as in congestive heart failure; the shock cannot be corre...
-
Product Concentrate of Trace Elements Solution for Infusion (I)
Trace element additive. For use in intravenous nutritional infusions to supplement the daily needs of adults for the trace elements as chromium, iron, molybdenum, zinc, copper, manganese and selenium.
-
Product Aseptic fill and finish
We offer solutions for companies that need CMO capacity concerning the aseptic filling of syringes and assembly and packaging of pre-filled syringes and auto-injectors. We will enhance your product by adding value through manufacturing if you require additional capacity or a complete technology transfer. q...
-
Product Polymer-based delivery systems
Polymer-based dosage forms continuously administer the drug to the patient for an extended period, varying from weeks to years. These controlled-release products are very suitable for specific indications, patient populations, and drugs due to the long duration of action, avoidance of first-pass metabolism...
-
Product High-potent Dosage Forms
We have the experience, expertise, and facilities at Sever Pharma Solutions to safely work with your high-potent substances. This includes manufacturing products containing APIs that are ranked in the highest occupational exposure bands (OEB) up to and including OEB6.
Our focus on safety starts ...
-
Product TTS - Transdermal Therapeutic System
Transdermal Therapeutic Systems (TTS) from LTS is a proven, yet flexible, ready to use platform designed to deliver a range of active substances.
TTS are pharmaceutical patches which are applied to the skin. The active substance enters into the bloodstream transdermally and acts where it's needed.
...
-
Product AdvaTab® Orally Disintegrating Tablets (ODTs)
AdvaTab® Orally Disintegrating Tablets (ODTs) incorporates coated or uncoated drug particles that are uniformly dispersed in a low-moisture, rapidly disintegrating matrix. Each ODT is formulated to achieve an acceptable taste and desired release profile. AdvaTab® technology is ideal for patients who ha...
-
Product Microcaps® Taste Masking Technology
Microcaps® Taste Masking Technology achieves uniform and efficient coating of drug particles by a combination of coacervation (phase separation) and spray coating to build polymeric membranes of varying porosity and thickness. The completely encapsulated particle allows for a pleasant mouthfeel wi...
-
Product Diffucaps® Customized Release Technology
Diffucaps® Customized Release Technology has the flexibility to incorporate functional, release-controlling polymers or protective coatings onto drug-layered cores, granules, or crystals. This allows for easy adjustment of both dosage strength and dissolution profile to achieve the desired in vivo phar...
-
Product MMTS (TM) Multi Mini Tablet System Customized Release Technology
MMTS TM Multi Mini Tablet System Customized Release Technology combines the simplicity of a tablet formulation with the flexibility of multiparticulate dosage forms with high drug-loading capability. Adare has developed Ultra Microtablets—a smaller standard of tablets targeting diameters in the range o...
-
Product TOPS - Topical Patch Systems
Topical Patch Systems (TOPS) from LTS is a flexible, ready to use platform designed to deliver a range of topical therapies, while avoiding first-pass metabolism and improving drug efficacy. With teams of experts on hand across the globe, we are here to support your next innovative drug delivery developmen...
-
Product MAP - Micro Array Patches
Micro Array Patches (MAP) from LTS are designed to enhance delivery through the intradermal route delivering a wide range of APIs including biologics and vaccines. MAP use tiny needles that penetrate the top layer of skin, dissolve and release their active ingredients. Our MAP platform is based on dissolva...
-
Product Development Oral Solid Dosage forms
XEDEV provides wide range of technologies in order to develop your oral solid dosage forms. • Spray-drying • Granulation • Tabletting • Encapsulation • Coating Contact us for more information.
-
Product Formulation and process development
XEDEV is specialized in formulation and process development.
Do you have an NCE that needs to be formulated in a solid dosage form? XEDEV can help you through drying, granulation, tabletting or coating processes.
We are using a stepwise approach to transfer your material to...
-
Product Drug Product
At Recipharm, we offer drug product development services for all common dosage forms. Our team can manage the complexity of your project, helping you find the best solution whether you are looking for development support to take your product to first-in-human (FIH) studies or to advance your product to mar...
-
Product Dissolution Testing - R&D services
For us dissolution testing is never a routine test but an essential tool in pharmaceutical formulation development to:
• evaluate the physicochemical properties of drug candidates to select the most appropriate solid form for further development (pre-formulation)
• compare pro...
-
Product Analytical research & development
Our analytical experts know what it takes to bring a pharmaceutical product to the market, from discovery, feasibility to GMP. Having worked with more than 100 unique molecules and even more formulations, we may state that we have faced the most difficult CMC challenges that are involved.
...
-
Product Excipient Quality Testing and Selection Services
Excipient testing, composition and variabilityExcipients are either natural / naturally derived or synthetic / semi-synthetic. In all cases the exipients are obtained through chemical processing of a raw material source that usually has an animal, vegetable or mineral origin.
...
-
Product Research & Development of medical devices
We provide a complete and comprehensive range of services from A to Z: from product idea, through development and research, certification, registration and product delivery to the customerWith us, you can easily launch a new medical pharmaceutical device on the market or adapt an existing on...
-
Product Late Stage Development - Integrated Programs
.Accelerating products through to commercial manufacture - Speed the journey from proof-of-concept to commercial launch. The journey from proof-of-concept to commercial launch can be complex. Speed is critical but it shouldn’t come at the expense of product quality. Creating patient-centric formulatio...
-
Product R&D and Industrialization
We specialise in research and development in the ophthalmic field for multinationals, Italian as well as international pharmaceutical companies. For them, we develop, fine-tune and manufacture, in aseptic conditions, pharmaceuticals and sterile medical devices for topical use in differen...
-
Product Oral gels, oral pastes, and liquids
Liquid forms : oral gel, oral paste, liquid ... filled in specific packaging according to species, product, dosage, use : syringe, cartridge, airless pump bottle, glass bottle, plastic tube
-
Product Products for pets
Products for pets : tablets, powders, oral gels and pastes ... filled in specific packaging according to species, product, dosage, use : syringe, airless pump bottle, plastic tube, plastic bottle // Using animal orgin factors to enhance palability // Compliant to EU regulation
-
Product Products for livestock
Products for livestock : oral gels, oral pastes, liquids, powders, bolus, effervescent tablets ... filled in specific packaging according to species, product, dosage, use : plastic bottle, aluminium pouch, bag, bucket // Compliant to EU regulation
-
Product LANSOPRAZOLE Micro PELLETS 12.5%W/W: (Lansoprazole Enteric Coated Micro pellets 12.5%)
LANSOPRAZOLE Micro PELLETS 12.5%W/W: (Lansoprazole Enteric Coated Micro pellets 12.5%)
Physical Specifications: White to off while spherical pellets, Pellets Size: ASTM 30#50, ASTM 25#50
Release specification: As per latest USP std. including additional parameters i.e. ...
-
Product GLICLAZIDE S.R. TABLETS 30 mg - (New Product)
Valpharma provides a wide range of products which includes GLICLAZIDE S.R. TABLETS 30 mg for the therapy of maturity onset Diabetes Mellitus (non-insulin-dependant or Type II), where dietary management alone has been insufficient.Gliclazide is an oral hypoglycemic used for the control of blood glucos...
-
Product MELATONIN S.R. TABLETS 2 mg (New product)
Valpharma provides a wide range of pharmaceutical products among which MELATONIN S.R TABLETS 2 mg (a product under development).Melatonin S.R. tablets 2 mg is indicated as monotherapy for the short-term treatment of primary insomnia characterized by difficulty in getting to sleep and in keeping t...
-
Product Formulation of lyophilized products
A correct formulation means greater stability of the active ingredient and provides protection against the stress suffered during the lyophilization process itself.
The selection of the appropriate excipients is really important in the development of a freeze-dried product.
-
Product Lyophilization Process Development (Quality By Design approach)
With the aim of obtaining a product that meets the critical quality attributes, but with the most optimized freeze-drying process.
Our focus is the total control of the process. We develop freeze-drying processes in a previously defined design space: Quality By Design approach.
-
Product Out Licensing Dossiers
• Out Licensing • Technology Transfer • Co-Development / Profit Sharing with Technology Partners
API partners
Marketing Partners
&...
-
Product OCTAGONAL BLENDERS
Salient Features: -
· In Compliance with cGMP guidelines.
· Effective blending with help of mixing baffles.
· Qui...
-
Product ROLLCOMPACTOR - 200/200KG
Optional Features: -
⇛ AC Variable frequency drive for Rollers.
⇛ Water cooling system for roll and hopper.
⇛ Hydraulic power pack system for Rear Roller.
⇛ Ch...
-
Product cGMP Manufacturing - Bags, PFS, Vials - Exceptional Range of Sizes and Fill Volumes
August Bioservices can provide clinical and commercial manufacturing fill finish services - with supporting formulation development, scale up and analytical testing capabilities - across a wide range of injectable products, container sizes and fill volumes.
Vials - from 1/2 mL up to 500 mL
Syringes...
-
Product Biotech Pioneers - State of the Art CDMO in Nashville, TN
August Bioservices is a US-based CDMO offering end to end outsourced services that support our pharma and biotech clients' projects throughout the drug discovery, development and manufacturing lifecycle.
This piece highlights the company's $65 million expansion investment and the impact on the local...
-
Product SGM
Sugammadex is one of the greatest successes in the history of cyclodextrins. There is an increasing interest for this product and for the development of Sugammadex since the approval by the FDA resulted in a 3-4-fold increase in the global sales of the product. SGM is used in anesthesia as an injectable an...
-
Product NANZILON HAND RUB (Chlorhexidine Gluconate & Alcohol Skin Antiseptic Solution)
NANZILON HAND RUB
Contains :
Chlorhexidine Gluconate
Solution IP ...
-
Product NANZILON- HC (Hospital Concentrate)
NANZILON- HC
Contains :
Chlorhexidine Gluconate
Solution IP &nb...
-
Product POVINANZ SOLUTION (Povidone Iodine Solution)
POVINANZ SOLUTION
Composition :
Povidone Iodine IP 5% w/v
(Available Iodine: 0.5%w/v)

-
Product INSTA-ACT LIQUID (Surface & Equipment Disinfectant)
INSTA-ACT LIQUID
Each 100 gm Contains :
Ethanol IP &nbs...
-
Product ICL
• MAGNESIUM CARBONATE • MAGNESIUM OXIDE • MAGNESIUM HYDROXIDE • POTASSIUM CHLORIDE • SCORALITE • SCORALITE DC
-
Product Solvay
• DSS - Docusate Sodium (100%) • DSS GRANULAR - Docusate Sodium (85%) with Sodium Benzoate • DSS 50% - Docusate Sodium (50%) in PEG 400 • ANTAROX F 127 NF
-
Product Formulation Development
Glatt Pharmaceutical Services develops and produces solid pharmaceutical dosage forms. Our focus lies on multiparticulate systems such as pellets, micropellets and granules. Whether you are looking for optimal bioavailablity or taste masking, improved solubility or stabilization of the dosage form, we have...
-
Product Spray Drying - GMP, Cyto or Non-Cyto, Potent
CritiTech's range of Spray Drying services is what sets it apart from other CDMOs. From Proof of Concept through GMP scale production. Dedicated Cytotoxic and Non-Cytotoxic SD equipment and facilities. Ability to process potent materials. Other key features include:
• Spray-dried dispersions - API + p...
-
Product Purcision™ - Platform for Direct to Target Drugs
Purcision™ is a proven particle engineering technology used to develop new drugs and reformulate and repurpose existing drugs for multiple delivery systems.
• Purcision technology is different from other particle engineering technologies (CESS, RESS, spray drying, milling)
• Purcision technology ...
-
Product VELOXTAR technology
VELOXTAR technology
The technology contributes to improved patient compliance with remarkably fast disintegration.
-- Ultra rapid disintegration in 4-6 seconds in the oral
-- Utilizes dry tableting process
-- Good tablet strength and hygroscopic...
-
Product DYNO®-MILL
GRINDING & DISPERSION TECHNOLOGY
Wet grinding technology focuses on the controlled real size reduction, dispersion and deagglomeration of solid particles in a liquid medium. Particle finenesses in the micro to nanometer range can be achieved. For more than 60 years, WAB-GROUP&n...
-
Product QEdge - Enterprise Quality Management Suite
QEdge is an enterprise-wide Risk based, Quality management Software to ensure product Quality and regulatory compliance, and Audit Readiness.
·QEdge eQMS has configured 60 + Process
Data Integrity Part 11 (21 CFR Part 11, Annex 11) compliant Risk ...
-
Product Formulation development for generics
At DiHeSys, we develop inks and polymers - individually for every single active ingredient. The corresponding formulation development takes place primarily in cooperation with the pharmaceutical industry. This approach allows us to offer our customers a steadily growing range of formulations tailored to th...
-
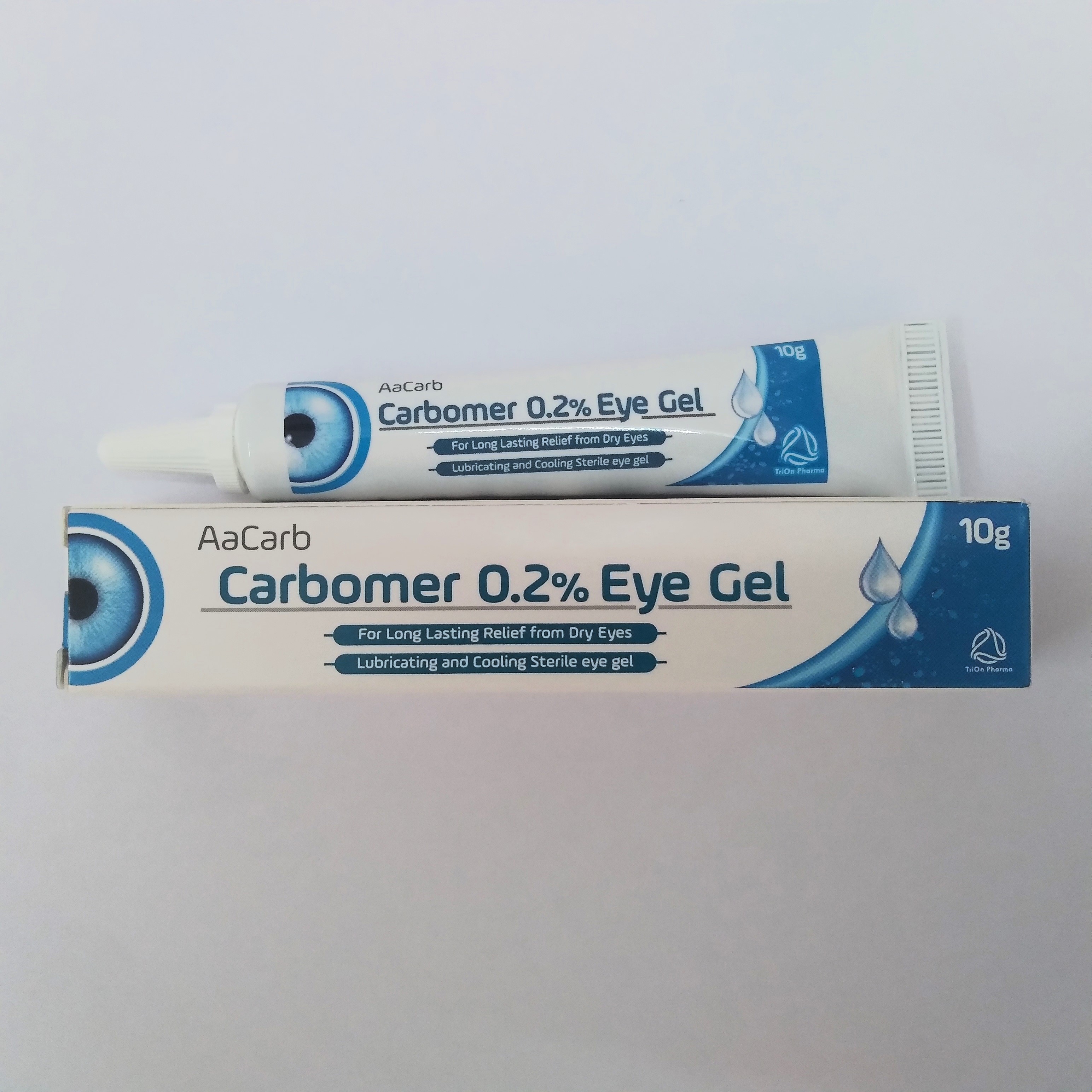
Product Carbomer & Hypromellose Lubricant Eye Gel Ophthalmic Night Time Ointment
Pioneer in manufacture of Ophthalmic Preparations especially Lubricant eye drops, Lubricant eye ointments and Lubricant eye gels. Night time and Day time lubricant products including Carbomer, Hypromellose, Carmellose and Sodium Hyaluronate with various combinations.
-
Product Paclitaxel Inj 30mg 100mg 300 mg 260mg
Paclitaxel Injection having 30 mg 100 mg 260 mg and 300mg this 4 strength available

-
Product Formulation Development Services
We at Neuheit can develop products for any market and perform tech transfer to our customer desired CMO. We specialize in handling complex generic product develop services.
-
Product Formulation Development
We devise and implement product specific development strategies capable of delivering high-quality formulations and robust manufacturing processes.
To achieve our clients’ goal, several studies are performed as a means of achieving deliverables assigned to certain distinct development phases...
-
Product Highly Potent API Drug Product Development
Highly potent API dedicated suite
The construction of a highly potent API laboratory to ensure operator and environmental safety has been initiated in 2022 to extend our in-house technical capabilities for new product developments.
The 100 sq.m. dedicated suite for highly potent produ...
-
Product Pre-formulation Profiling and Drug-ability Assessment
Pre-formulation Profiling and Drug-ability Assessment in the pharmaceutical industry involve rigorous testing and analysis to determine a drug candidate's physical, chemical, and biopharmaceutical properties. This critical step guides formulation development, optimizing drug delivery, and enhancing bioavai...
-
Product Formulation Development (NDA/ANDA)
Formulation Development is a crucial process in creating safe, effective, and market-ready medicines. Whether for New Drug Applications (NDAs) or Abbreviated New Drug Applications (ANDAs), this process involves optimizing drug composition, dosage forms, and delivery methods. By combining scientific experti...
-
Product Clinical And Product Development Services
Pre-formulation Development
Drugs are seldom administered to patients as pure compounds, instead they are formulated into medicinal products: but many that start their development journey don’t make it to market, failing for a variety of reasons from inefficacy or toxicity to commercial or manufac...
-
Product VetaFilmTM - Veterinary Oral Film Technology
VetaFilm™ is our proprietary veterinary oral film technology that provides significant market opportunities to improve drug delivery for companion animals. VetaFilm™ research and development services and veterinary oral films provide: • Dose placed directly on oral mucosa or on their f...
-
Product Biotest Human Serum Albumin
• Human Serum Albumin for the biotechnology and pharmaceutical industry • We offer a human serum albumin (HSA) product that complies with the regulatory requirements of multiple health authorities. • It is manufactured in a GMP compliant, state-of-the-art manufacturing plant and supplied und...
-
Product Formulation Development
Our ExpertiseTablets : IR, SR, ER, DR,ODTs, MUPS, Sublingual & Effervescent dosage forms
Capsules : Powder, Granules and Pellets filled in capsules
Powder and Granules : Sachets and Bottles
Oral Liquids : Solutions, Suspensions and Emulsions
Parenterals : Ophthalmic...
-
Product Drug Development
Service Overview Drug Development and Manufacturing • Development of solid, semi-solid and liquid formulations for oral, topical, nasal and parenteral application
• API characterisation and quality screening • Small batch manufacturing §Clinical trial batch manufacturing and distributio...
-
Product Containment & Aseptic Barrier Isolator Systems
n the global pharmaceutical market with the ever growing demands on materials, operator and environmental protection, consideration toward increasing standards of safety, sterility assurance and regulatory compliance has never been higher.
As applications expand into less traditional are...
-
Product Formulation Development
1. Experts in analytical chemistry, Pre- formulation, formulation and nutraceutical development and optimization, as well as analytical testing and development services.
2. Extensive experience working Specialty Complex Products, Potent Products & variety of dosage forms.
3. Pro...
-
Product GCP Services(Good Clinical Practices)
We offer various GCP services which can help the clients across globe, ranging from audits to monitoring services. We provide comprehensive GCP Quality Compliance services to the pharmaceutical industry. Our GCP consulting services are characterized by:
1) Knowledge: Various regulatory requi...
-
Product NanoAssemblr® Ignite™
Genomic medicines have a wide design space that requires optimization to select a lead candidate. The NanoAssemblr® Ignite™ is ideal for fine tuning process parameters and compositions for further scale up to the clinic.
Easily scale between 1mL and 20mL formulations on the Ignite™. Optimiz...
-
Product NanoAssemblr® Blaze™
The NanoAssemblr® Blaze™ allows rapid scaling of nanoparticle formulations for late pre-clinical development. The system can manufacture between 10 mL and 10 L of formulation which allows researchers to use the Blaze for preclinical toxicology testing, and for early Chemistry, Manufacturing and C...
-
Product Delivery systems / formulations for drugs with low water solubility
Develop delivery systems for drugs with low water solubility
-
Product Development Service
Lomapharm GmbH provides wide range of services which includes development service. It provides the whole range of control services - from raw material testing to stability testing according to GMP and ICH regulations. One of the main areas of competence and experience is also available for galenic dev...
-
Product Colony Counter and Documentation System with 21 CFR Part 11 Compliance
Colony Counter and Documentation System with 21 CFR Part II Compliance Software • Automated colony count in seconds. • Documentation of Colony for future Reference. • Minimum operator interaction • Meets FDA 21 CFR Part 11 Compliance • Compact system utilizes incident, tra...
-
Product 21 CFR Part 11 Compliance Microscope with Image analysis & Documentation Systems as per USP 695 & 776
21 CFR Part 11 Compliance Image analysis and documentation Systems for documenting to meet USFDA Guidelines for
• Images from Polarized Light Microscope • SEM & TEM Image Analysis • Inspection System • Image documentation for microbiology like gram staining

-
Product Filter Paper Scanning system for Contamination Testing in Injectable, Ophthalmic & Extraneous Matter for API
• Filter Paper Scanning Microscope to meet extraneous matter Analysis • Filter Paper Scanning Microscope with 21CFR Part II Compliance to meet USP 788 for Particle Count in Injectable • Filter Paper Scanning Microscope with 21CFR Part II Compliance to meet USP 789 for Particle Count in Ophth...
-
Product Rapid Mixer Granulation
RAPID MIXER GRANULATOR Machine is used for homogeneous mixing of granulation powder used for production of tablets. It is also used for process of wet mixing & fast dry, humidifying of the powder in Chemical, Cosmetic, Food, Plastic Pharmaceutical, General Mixing Industries, etc.
Rapid Mix...
-
Product Cosmetic Ampoules
Skin ampoules are the latest top trend. Single-dose with a high concentration of active ingredients, they work an instant effect on the skin. The volume chosen is ideal for using half the ampoule in the morning and half at night. They must always be applied to clean skin, and can be spread by gently m...
-
Product Cosmetic products
Cosmetic products for facial and body application.
Available formats: creams, gels, serums, masks, scrubs, photoprotectors, ampoules, etc.
Available applications: anti-ageing, firming, moisturising, anti-wrinkle, soothing, anti-inflammatory, eye contour, anti-acne, anti-ox...
-
Product Chemical Peelings
Professional use. Chemical peels are topical treatments, mainly formulated on acids, designed to eliminate in a controlled way the most superficial layers of the skin, stimulating cell renewal and improving significantly its appearance. The most remarkable clinical effect is rejuvenation.
-
Product Capsules – Food Supplements
Inalme offers a wide range of customizable types of capsules. The entire manufacturing process is fully automated, from the moment the raw materials start getting processed to the end, when the finished product gets packaged, ready for shipping.
Capsules are available in the following fo...
-
Product Cleansers and washes - Cosmetics
Inalme offers a wide range of customizable types of primary packaging for cleansers and washes. The entire manufacturing process is fully automated, from the moment the raw materials start getting processed to the end, when the finished product gets packaged, ready for shipping.
The avai...
-
Product Final sterilisation (vials and ampoules)
Prasfarma S L offers dosage forms, totally specialized in oncologic and HPAPI products which includes final sterilisation. It belongs to Manufacturing of sterile forms (small volume) category. Contact us for more information.
-
Product Tablets and film coated tablets
Prasfarma S L offers dosage forms, totally specialized in oncologic and HPAPI products which includes film coated tablets. It belongs to Manufacturing of oral solid cytotoxic drugs category. Contact us for more information.
-
Product Pre-Formulation / Formulation
Asymchem understands that early phase work focuses on learning as much as possible about a drug compound. Our experience across numerous chemical classes and multiple dosage forms, and our ability to develop analytical methods to assess chemical identity, purity, compatibility, and stability, makes us a na...
-
Product Pharma Product Development
We develop oral tablet formulations, oral powder-filled capsule formulations, oral and topical solutions, and oral suspension formulations.
Sovereign offers:
• DEA License Schedule I-V • extensive experience of over 100 APIs • a high potency pharma suite • and solid and liquid dose manu...
-
Product Contract Development and Manufacturing
We are a Pharmaceutical contract development and manufacturing company authorised for the preparation of medicinal products and medical devices in liquid, suspension, gel and cream form, lyophilisates and ointments. Our services include formula development, pilot studies in the laborato...
-
Product R&D and Industrialization
We specialise in research and development in the ophthalmic field for multinationals, Italian as well as international pharmaceutical companies. For them, we develop, fine-tune and manufacture, in aseptic conditions, pharmaceuticals and sterile medical devices for topical use in different fo...
-
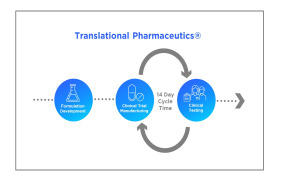
Product Translational Pharmaceutics
Translational Pharmaceutics®, accelerates development by integrating formulation development, real-time manufacturing and clinical testing. This integrated approach is proven to reduce timelines by more than 12 months and lower R&D costs by more than $100 million. Over the past decade ...
-
Product Formulation Development
From preclinical and first-in-human (FIH) dosage forms to optimization of your drug product for late-stage development and market following clinical evaluation, we work with you to develop the most appropriate formulation based on the physicochemical and biopharmaceutics properties of your drug molecule, t...
-
Product Pharmaceutical Excipients
The world’s most authoritative source of information on excipients
The Handbook of Pharmaceutical Excipients is the definitive summary of excipient specifications including pharmacopeial information and their regulatory status, providing you with a one stop resource when researching an e...
Upcoming Events
-
-
-
CPHI & PMEC India 2024
India Expo Centre, Greater Noida, Delhi NCR
26 Nov 2024 - 28 Nov 2024
Pharmaceutical Industry Webinars
-
Webinar Fragment-Based Oligonucleotide and Oligopeptide Synthesis
-
30th Jul 2023
-
4pm CET / 10am EST
-
-
Webinar GMP Rationale for Sterile High-Potency/Toxic Pharmaceuticals
-
18th June 2024
-
4pm CET / 10am EST
-
-
Webinar Unlocking Opportunities in the Growing Pharma Landscape of The Middle East
-
5th June 2024
-
3pm CET / 9am EST
-
-
Webinar Exploring Technological Trends in the Future of Pharmaceutical Manufacturing
-
23rd May 2024
-
4pm CET / 10am EST
-
-
Webinar Achieving Manufacturing Excellence Through Digital Transformation
-
16th April 2024
-
4pm CET / 10am EST
-
-
Webinar Made in Africa: What’s Driving Pharma Manufacturing
-
28th March 2024
-
4pm CET / 10am EST
-
-
Webinar Case Study: Risk Management for Annex 1 Sterile Production EMS
-
28th February 2024
-
4pm CET / 10am EST
-
-
Webinar Innovative Strategies for B2B Pharma Marketeers: Driving Value through Content
-
20th February 2024
-
4pm CET / 10am EST
-
-
Webinar Revolutionizing Pharma: Data and AI Unleashed
-
18th January 2024
-
4pm CET / 10am EST
-
-
Webinar Optimal Temperature: Elevating Biologics Cold Chain Excellence
-
16th January 2024
-
4pm CET / 10am EST
-
-
Webinar Market Outlook – The Biggest Pharma Trends of 2024
-
12th December 2023
-
4pm CET / 10am EST
-
-
Webinar The Next Frontier – Emerging Opportunities in the LATAM Pharma Market
-
21st November 2023
-
4pm CET / 10am EST
-
-
Webinar Vistamaxx™ MED - imagine the possibilities for healthcare product performance
-
10th October, 2023
-
4pm CET / 10am EST
-
-
Webinar Co-processing: A Multifaceted Approach for Enhancing Density & Powder Flow
-
19th September 2023
-
4pm CET / 10am EST
-
-
Webinar The Outlook for Cell & Gene Therapy Manufacturing
-
28th June 2023
-
4pm CET / 10am EST
-
-
Webinar Contract Packaging Outlook: Growth Trends within the Commercial Packaging Sector
-
25th May 2023
-
4pm CET / 10am EST
-
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance




























































































































































.jpg)




























































.png)

.png)



.jpg)