Biopharma News
Biopharma news
-
News FDB to triple UK microbial production capacity as Fujifilm commits to $83 million investment
Fujifilm Diosynth Biotechnologies will triple microbial production capacity at its UK Teesside facility after joint owner Fujifilm Corporation pledged to invest 9 billion yen (USD83 million) in the biologics focused CDMO. -
News Oxford Biomedica signs LSA with Juno Therapeutics
Cell and gene focused CDMO Oxford Biomedica (OXB) has signed a licence and clinical supply agreement which could be worth up to USD227 million with Juno Therapeutics, the firms announced Wednesday. -
News What Impact will Coronavirus have on Pharma Supply Chains?
The worldwide inter-dependency of pharmaceutical manufacturing is highlighted by the ongoing Coronavirus situation. How can the global supply of diagnostic kits, drugs, medical supplies, and equipment be sustained to treat patients in the face of suppl... -
News Vaxxas HD-MAP to deliver vaccines directly to the skin
Company awarded US$5 million grant for clinical study of measles and rubella vaccination using Vaxxas’ high-density, micro-array patch. -
News Artificial Intelligence: Moving beyond efficiency gains in drug development
Is the pharma industry making the most of AI’s potential when it comes to accelerating and enhancing pharmaceutical drug development? -
News Unlocking the potential of cannabinoid-derived drugs
Following the 2018 FDA approval of GW Pharmaceuticals’ Epidiolex®, there has been increased interest in the development of cannabinoid-derived drug substances for innovative new therapies. We examine the state of the market and future potenti... -
News Sartorius simplifies biologics production with new bioreactor
Company launches BIOSTAT STR Generation 3 bioreactor with BIOBRAIN automation platform. -
News Potential first-in-class treatment reaches significant milestone
The US Food and Drug Administration (FDA) accepts for review Pfizer and Eli Lilly's Biologics License Application (BLA) for tanezumab — a non-opioid treatment for patients with chronic pain due to moderate-to-severe osteoarthritis. -
News Trump and pharma execs to discuss progress on COVID-19 cure
The White House task force and the US President will meet drug makers this afternoon to discuss "progress on a vaccine and cure". -
News Packaging Toward a Sustainable Future
With the world’s attitude to tackling climate change arguably the global number one hot topic of 2019, the issue of sustainability is an emerging theme in pharmaceutical packaging. -
News BIA Separations signs agreement to commercialise novel elution technology
Agreement gives BIA Separations access to proprietary technology to better preserve integrity, infectivity and potency of immunoaffinity-purified viral vectors for gene therapy. -
News New Sartorius QbD tool supports faster Raman model building
Unlocking the potential of Raman spectroscopy as a high throughput analytical technique for monitoring cell culture in mini bioreactors. -
News Pipelines, Economics and IP: How Bioprocessing Tech Firms Assess Markets
Identifying areas of unmet need to guide R&D strategy and shape drug pipelines -
News Univercells' €50M investment to speed delivery of manufacturing tech
The investment will support continued expansion into the fast-growing gene therapy segment, including new developments that will enable a range of best-in-class solutions for viral manufacturing. -
News Sphere Fluidics expands to increase supply of surfactant for droplet microfluidics
Investment in resources to meet demand for large-scale supply of patented biocompatible surfactant. -
News Sartorius sharpens its brand focus
The company aims to simplify its customers’ work and thus help them to achieve medical progress and make innovative medicines available faster. -
News US Pharma Outlook: Ten Trends to Look Out For in 2020
With CPHI North America fast approaching, we asked several industry experts what they see as the main trends emerging across the US pharma supply chain this year. -
News Inovio and Beijing Advaccine to advance vaccine against Coronavirus
Agreement will facilitate clinical trial translations in China. -
News First human trial of monoclonal antibody to prevent malaria opens
If proven safe and effective, mAb CIS43LS might be used prophylactically by tourists, medical workers or military personnel who travel to areas where malaria is common. -
News Merck invests in biotech development facility in Switzerland
€250 million to be invested during the 2019-2022 period in new facility bridging research and manufacturing. -
News Grey Wolf Therapeutics secures funding for cancer therapies development
The company targets ERAP antigen presentation pathways with the aim of ‘illuminating’ non-responsive tumours for attack and destruction by the immune system. -
News Wuxi to acquire Bayer final drug product manufacturing site
First drug product facility in Europe to complement WuXi Biologics’ existing commercial manufacturing capacities. -
News BioMarin gets go-ahead to start gene therapy clinical trials
BMN 307 represents a potential third PKU treatment option from BioMarin and its second gene therapy clinical program. -
News Intensification and Consistency: Driving Biomanufacturing Innovation
Biopharma’s desire for fast, cheap production and cell and gene therapy firms’ need for consistency is shaping tech development in the sector. -
News Partnership formed to develop and commercialise ready-to-use medicine
The product being developed will use proprietary drug formulation technology platform Arestat to deliver new reformulations of existing, complex products. -
News UCB to build new biotech manufacturing plant in Belgium
Capacity to serve global late-stage development and commercial manufacturing of monoclonal antibody drug substance to support new product launches. -
News Sartorius tests and develops AI in its products and platforms
Customers and partners look set to benefit from reduced development times and costs of medical drugs. -
News Pharma Outlook: 10 Trends to Look Out For in 2020
We asked ten industry experts for their thoughts on some of the emerging trends for the pharmaceutical supply chain as the industry enters 2020... -
News 'Niche' CMO invests in UK facility to expand viral vector sterile manufacturing
CMO's customer base has grown by more than 25% in 2019 alone, and investment and demand for services is estimated to increase output by another 25-30% in 2020. -
News Sanofi bolsters its immuno-oncology pipeline for $2.5B
Lead asset THOR-707 being explored across multiple solid tumor types alone and in combination with immune checkpoint inhibitors and other future IO combinations. -
News Eli Lilly unveils shared innovation laboratory in San Francisco
Lilly Gateway Labs will provide biotech companies direct access to Lilly scientists and expertise. -
News New single-use mixing system offers rapid and homogenous mixing
ClearMixx performs liquid/liquid and powder/liquid mixing, with a dispersion plate able to efficiently mix the most challenging buffer, media and biopharmaceutical ingredients. -
News India pharma forecast to grow strongly in 2020
The rise in exports growth potential is believed to be in response to concerted reforms by the CDSCO and industry quality improvements in the last few years. -
News GVK Bio continues to see growth tailwinds for 2020 thanks to a ‘crescendo of factors’
China-US trade challenges has led to de-risking by pharma companies. -
News Winners of the 16th Pharma Awards
Companies and individuals from across the whole pharma supply chain recognised for their excellence and commitment. -
News CDMOs to benefit from rising BTDs, orphan drugs and fast track status therapies
But personalized medicines will require new logistics and manufacturing systems. -
News Europe predicted to surpass the US in biologic manufacturing capacity by 2023
Demand for biological manufacturing is growing faster than capacity growth. -
News Legacy recruits heavily to meet growing steriles demand
Company to reach 250 employees by year end to meet growing drug product manufacturing demand. -
News China on course for 'massive' bio capacity shortfall
A shortage of qualified personnel may be a drag factor in cell and gene therapies; in China 100,00L of capacity will need to be added every year to meet bio demand. -
News Evonetix expands to develop its desktop DNA synthesis platform
15,000 sq. ft of laboratory and office space will enable continued growth and development of the company’s integrated desktop platform for DNA synthesis. -
News The talk of the show?
Outsourcing, biologics, generics and patient compliance look set to get tongues wagging at this year's CPHI WW. -
News Sartorius Stedim Biotech launches new Sartocheck 5 Plus filter tester
Highest level of data integrity as requested by regulatory authorities. -
News Sanofi opens its first digitally-enabled, continuous manufacturing facility
One of the first digital manufacturing facilities in the world to use continuous, intensified biologics production technology. -
News Discover Roquette’s latest innovations and our new Biopharma Innovation Center!
Roquette works closely with customers in the development of innovative pharmaceutical formulations and new biopharma solutions. -
News Industrial-scale manufacture of therapeutic exosomes
Coordinated purification process development service accelerates progress to clinical trials, and scale-up manufacturing. -
News Data on lead ADC demonstrates effective tumour regression
IKS01 shows marked anti-tumour efficacy in pre-clinical models of ovarian and lung cancer. -
News Arctoris secures funding to advance novel robotic drug discovery platform
Platform will enable scientists and biotechnology entrepreneurs worldwide to make discoveries faster and more efficiently, -
News GSK invests $120 million in next-generation US biopharma manufacturing facility
Part of nearly $400 million in US manufacturing investments to deliver on the company’s pipeline and bring medicines to patients faster. -
News FUJIFILM Irvine Scientific launches BalanCD Gal Supplement for biotherapeutic development
Delivers enhanced galactosylation for improved protein quality, and antibody binding and function. -
News Sandoz enters global deal to commercialise proposed biosimilar natalizumab
Worldwide agreement with Polpharma gives Sandoz commercialization rights to proposed biosimilar natalizumab for relapsing-remitting multiple sclerosis. -
News Rapid adoption of Carterra’s LSA instrument drives European expansion
Company's antibody screening platform minimizes the risk of missing a blockbuster. -
News HALIX starts operational production with new cGMP facility in Q4-2019
The five-level production facility contains a state-of-the-art manufacturing line for viral vaccines and viral vectors and a separate protein manufacturing area with a capacity up to 1,000-L single-use bioreactors. -
News Delivering complete aseptic vial handling solutions
Bringing freeze drying and vial washing/sterilizing technologies together with advanced and comprehensive vial and syringe filling capabilities. -
News Sartorius launches new services for mammalian cell bank manufacturing
Integrated package of new and established services saves time and minimizes risks. -
News Needle free: Erase wrinkles without injection
Meet REVOLUTION BEYOND INNOVATION at Cphi Worldwide 2019, Booth No. 42C06 -
News New state-of-the-art cleanroom boosts production of anticancer drugs
The GMP facility has been specifically designed to deliver the next generation of anticancer blockbuster ADC drugs for patients globally. -
News Eisai collaborates with University of Dundee on cancer drug discovery
It is hoped that research into PROTACs will lead to new drug discoveries for proteins present in cancer, which are difficult to treat with conventional small molecule inhibitors. -
News Fujifilm Irvine Scientific to open new cell culture media manufacturing site in Europe
The space will support cGMP manufacturing of animal component-free, dry powder media, liquid media, and downstream bioprocessing liquids. -
News Fresh data on protein interactions in Alzheimer’s disease
Demonstrates the unique ability of diffusional sizing to assess protein binding in-solution and for difficult-to-study systems. -
News Cyclolab Ltd strengthens its position in the market
Cyclolab Ltd. is an R&D and cGMP manufacturing company focusing on all aspects of cyclodextrin science, manufacturing and applications for cyclodextrin, Dexolve, Sugammadex, formulation development, custom synthesis, Niemann Pick C. -
News New ambr 250 modular bioreactor vessel for cell and gene therapy applications
Designed for gentle stirring and optimum growth of cell lines. -
News New-generation incubator brings the latest technology to mid-capacity microplate cell culture
New Thermo Scientific Cytomat 2 C-LiN Series Automated Incubator delivers robust and reliable culture solution for cell-based pharmaceutical applications. -
News AbbVie to acquire Allergan in transformative move
Transaction will create a leading biopharmaceutical company with approximately $48 billion in combined 2019 revenue. -
News HCP Kit for automated impurity analysis of biotherapeutics
Ready-to-use immunoassay kit increases analytical output and productivity in bioprocess workflows. -
News Inaugural Bio Integrates conference highlights industry's inefficiency in developing products
Industry leaders give voice to issues and trends shaping the biotech sector, including the importance of collaboration. -
News Affordable measles and rubella vaccines using NevoLine manufacturing platform
Univercells' propriety bioproduction platform has already proven success. -
News Sphere Fluidics secures funding to support its single cell analysis system
Funding will also be used to further expand operations in the UK and US. -
News CPHI China opens with analysis pointing to a surge in growth in 2019
Regulatory reforms and harmonisation reported as key drivers of industry in China. -
News Catalent completes acquisition of gene therapy leader
Acquisition of Paragon Bioservices brings specialized expertise in adeno-associated virus and will enhance Catalent’s biologics business and end-to-end integrated biopharmaceutical solutions for customers. -
News Vibalogics increases capacity with a new 200-L line for virus manufacturing
Company has also invested in an Äkta-ready chromatography system for downstream processing and liquid handling equipment to deal with increased volumes. -
News New cell culture microbioreactor system improves clone selection
SSB's new generation of ambr 15 supports cell and gene therapy applications, including HEK293 for viral vector production, T-cells, iPSCs and other immune-derived cell lines. -
News Surge of Indian biosimilars market forecast in 2019
India predicted to be one of the world’s ‘fastest growing bio’ hubs in 2019, fuelled by new biosimilars production. -
News Isotype-specific secondary antibodies for improved signal detection
Offers an alternative to cross-adsorbed secondary antibodies when absolute specificity is required. -
News New China-based biotechs fueling growth in manufacturing across China
New guidelines will see poorer quality manufacturers drop out of the market. -
News 'Drugs from bugs' joint venture
Lonza and Chr. Hansen create a strategic joint venture to become the partner of choice for developing and manufacturing live biotherapeutic products for pharma and biotech customers. -
News ChargePoint Technology launches single-use technology as part of powder transfer solutions portfolio
Company's hybrid solution claims superior performance compared with tradition single-use technologies. -
News Sartorius integrates online biomass measurement to its ambr systems for microbial applications
Scientists to rapidly obtain detailed process understanding and control over their microbial cultures. -
News Bringing next generation single-use sensor technologies to the life science market
Pall and Broadley-James’ combined expertise proves complementary in addressing critical customer pain points in modern bioprocessing. -
News Novel systems for membrane chromatography
Optimally run membrane chromatography processes will provide higher productivity, smaller-scale operations and increased robustness. -
News Biogen acquisition boosts its ophthalmology pipeline
Nightstar Therapeutics has two potentially first-in-class mid- to late-stage clinical assets as well as preclinical programs. -
News Japan solid dose market predicted to bloom in 2019
Executives predict Japan to be the second fastest growing mature market for solid dose drugs in 2019, and the second fastest overall country for biologicals growth. -
News Partnership expands to develop a new treatment for pachyonychia congenita
MedPharm and Palvella Therapeutics hope to develop the first approved treatment for PC in the US and Europe. -
News Andrew Alliance and Sartorius collaborate to provide software-connected pipettes for life science research
Researchers to benefit from an innovative software-connected pipetting system, bringing improved reproducibility and traceability of experiments to life-science laboratories. -
News Hitachi Chemical to acquire apceth Biopharma
Acquisition will strengthen Hitachi's presence in the second-largest cell and gene therapy market in the world. -
News Sartorius joins the community of the National Institute for Innovation in Manufacturing Biopharmaceuticals
Company hopes to contribute substantially to further optimizing and accelerating the current manufacturing processes in the biopharmaceutical industry. -
News Particle Sciences partners with Encube Ethicals to develop novel vaginal rings
The partnership will focus on rings for the administration of existing approved drugs, improving drug effectiveness and patient compliance. -
News SGS expands testing capabilities at its Glasgow facility
Clients will benefit from a fully comprehensive range of validated biosafety methods to support cell bank and viral vaccine manufacturing and lot release of drug product. -
News TC BioPharm creates allogeneic cell banks for CAR-T cancer therapy products
The first completed bio-banks will be used to develop more cost-effective, safe and efficacious cancer treatments. -
News Alvotech completes US $300 million financing deal
Fuels growth and biosimilar development in a fast-growing market. -
News Sartorius introduces BIOSTAT RM TX single-use bioreactor
Flexsafe RM TX bags enable reliable process performance for optimal cell growth. -
News Univercells introduces breakthrough vaccine manufacturing platform
The automated NevoLine bioproduction system that facilitates safer, faster and closed bioprocessing in a much smaller footprint. -
News Allergan to establish R&D presence in Cambridge, Massachusetts
The new, strategic presence will allow the company to more easily interact and engage with venture firms and start ups in the area. -
News Expedeon signs supply and license agreement for is Lightning-Link technology
The technology will enable Cell Guidance Systems to manufacture TRIFic detection assays on-demand with highly reproducible and scalable results. -
News BMS and Celgene merge to create premier innovative biopharma company
Significantly expands Phase III assets with six expected near-term product launches, representing greater than $15 billion in revenue potential. -
News Pall opens Center of Excellence in Shanghai
The CoE showcases the latest end-to-end technological solutions for biomanufacturing. -
News New gene therapy manufacturing facility for Orchard Therapeutics
Enhances company's capacity to develop and deliver lentiviral vector and gene-corrected hematopoetic stem cells for wide range of diseases on a global scale. -
News Grant winners to investigate continuous manufacturing for gene therapies
Cobra Biologics, Pall, and the Cell and Gene Therapy Catapult share £1.5 million grant from the UK's innovation agency. -
News ADC Bio secures additional equity round investment
Extra funding for strategic move into downstream formulation and fill finish capabilities and full exploitation of Lock-Release as new ADC manufacturing paradigm. -
News SSB and Lonza modify relationship for supply of cell culture media
Sartorius Stedim Biotech will continue to offer Lonza media and buffer products but under a non-exclusive agreement. -
News bioLIVE to merge with BioProduction Congress for 2019 event
BioProduction’s content platform will be added to the bioLIVE exhibition, creating one of the world’s largest event hubs for the bio industry. -
News Sandoz pulls the plug on US biosimilar rituximab
Company will focus on robust biosimilar portfolio for unmet access and sustainability needs. -
News Three-way collaboration to advance gene therapy
Partnership aims to increase the robustness and reduce costs for the manufacturing of AAV vectors. -
News Bioconjugation expertise and increased productivity in bio over next 5 years
Smaller bioreactors and high-density perfusion techniques needed as manufacturing makes significant efficiency improvements. -
News Alvotech receives manufacturing licence for its biopharmaceutical facility
Facility to focus on developing and manufacturing the company's biosimilar portfolio. -
News Complete product lifecycle management in one location
Lonza expansion includes drug substance development, and drug substance and drug product manufacturing. -
News BIA Separations expands with new upstream processing facility
Provides single source of expertise for scaled production of complex biologics for gene therapy and vaccines. -
News Partnership to deliver integrated, off-the-shelf biosimilar manufacturing solutions
Pall and Aetos to advance the market impact of biosimilars and deliver lower-priced, high-quality options to end users. -
News New SGS biopharmaceutical testing capabilities in Illinois
The new services will focus on quality control analysis and stability testing of biopharmaceuticals. -
News Novartis to divest the Sandoz US dermatology business and generic US oral solids portfolio
The Sandoz US portfolios to be sold to Aurobindo include approximately 300 products, as well as additional development projects. -
News Sartorius and Repligen partner to introduce next-generation perfusion-enabled bioreactors
Customers to benefit from simplified, scalable solutions for intensified cell culture. -
News Plasticell wins military contract to develop regenerative medicines for the battlefield
Company to use its combinatorial stem cell screening platform to develop technologies for the conversion of pluripotent stem cells into platelets. -
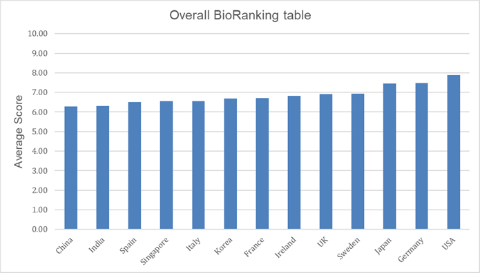
News Bio processing and manufacturing league table ranks US top and China bottom
US, Germany and Japan revealed as tier one nations in all categories with Sweden ranking the best of the rest. -
News New biopharmaceutical testing laboratory to open in Geneva
SGS's expansion will enable the service provider to offer a full ICH Q6B physico-chemical characterization of biological products. -
News Exosome isolation manufacturing and characterization
New service to reliably and reproducibly isolate exosomes from almost any biofluid. -
News Oxford Genetics signs major supply and licensing agreement for CRISPR engineered mammalian cell lines
Company moves away from manual processing in favour of automated, scalable platforms. -
News Extended distribution agreement enables customers to continuously improve their bioprocesses
Agreement includes Pall's next-generation Kaneka KanCapA 3G sorbent for the primary capture of mAbs from clarified cell culture. -
News Amgen breaks ground on next-generation biomanufacturing plant in Rhode Island
The plant will be first-of-its-kind in the US. -
News Partnership struck to aid bio manufacturers
Pall and BioSciencesCorp unite to provide "total solutions" for today's drug manufacturers. -
News WuXi STA and Antengene sign development and manufacturing agreement
WuXi STA chosen for its end-to-end CMC platform for new drug development. -
News New screening company exploits the latest advances in microfluidics and 3D culture
The ability to micro size drug-cell interactions will allow pharmaceutical and biotech companies to do 100x more testing for the same money spent as well as increasing productivity.
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance


.jpg)

.jpg)

.jpg)
.jpg)

.jpg)

.jpg)



.jpg)
.jpg)
.jpg)
.jpg)




.jpg)


.jpg)
.jpg)
.jpg)
.jpg)


.jpg)
.jpg)


%20(1).jpg)










.jpg)
.jpg)





.jpg)






.jpg)
.png)



%20(2).jpg)
%20(2).jpg)


.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
%20(1).jpg)








.jpg)