Cambrex completes $50 million investment in large-scale API manufacturing and storage
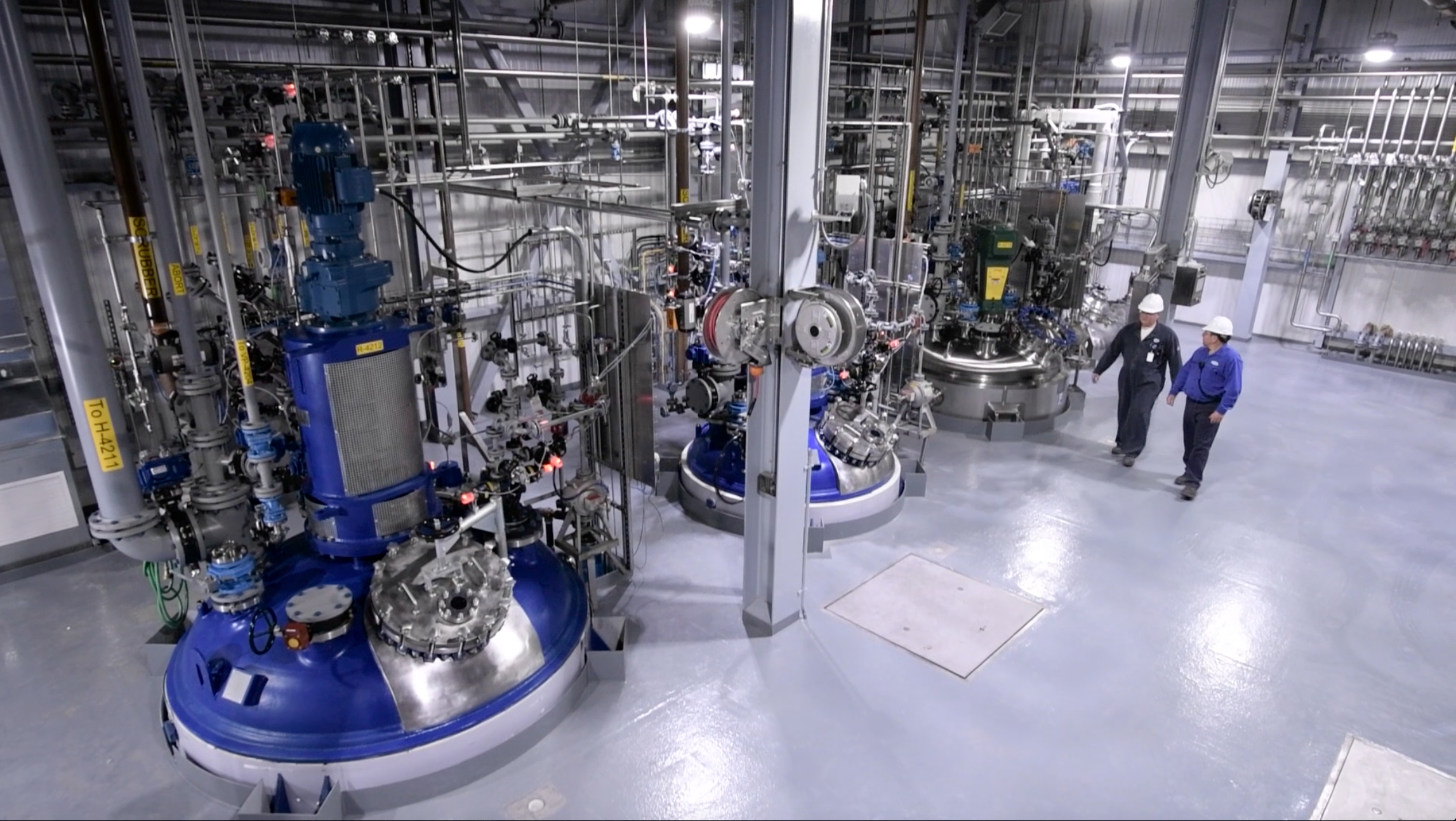
The company's investment reflects the strong market demand for small molecule APIs.
Cambrex has announced the completion and validation of a $50 million state-of-the-art production and warehousing expansion at its cGMP Charles City, Iowa site. The investment in capacity and capabilities was made in reflection of strong market demand for small molecule APIs and high utilization of Cambrex’s existing large-scale assets.
The new 7,500 sq. ft. multi-purpose manufacturing facility will initially add a total of 70 m3 of glass-lined and Hastelloy reactors ranging in size from 7 m3 to 16 m3, along with 6m 2 Hastelloy agitated filter dryers to provide a flexible, multi-purpose configuration, and will be capable of handling potent APIs at an Occupational Exposure Limit (OEL) of down to 1 μg/m3. The facility complements the three existing large scale manufacturing facilities at the Charles City site.
The 36,000 sq. ft. cGMP warehouse provides general cGMP storage for 2,720 pallets and segregated 2–8°C refrigerated storage for 360 pallet spaces and features Distributed Control System monitoring and control for temperature and humidity. The facility employs a barcode system for automated bin location and has a dedicated sampling room with appropriate extraction and handling for flammable materials.
In addition, a further 7,500 sq. ft. manufacturing shell has been constructed, which will be fitted out to customer specification.
“There is high demand for US based suppliers with large scale cGMP contract manufacturing capacity and world class quality systems,” commented Steve Klosk, CEO of Cambrex. He added: “We have invested over $125 million at the Charles City site since we acquired it in 1991 and believe that this expansion, combined with a very talented and experienced team, provides an ongoing foundation for our customers’ small molecule product manufacturing needs while supporting our goal of bringing our customers’ products to market quickly.”
Related News
-
News Eli Lilly gets ready to launch five new drugs in 2023
Eli Lilly, the American pharmaceutical company (IN, USA) are gearing up for a big year ahead, with hopes to launch five new drugs and capitalise on growing obesity and Alzheimer’s disease markets. -
News Amgen buys Horizon for $27.8 billion in bold step into the rare disease market
Amgen Inc buys pharmaceutical company Horizon Therapeutics in a multibillion-dollar deal, in hopes to capitalise on it's portfolio of drugs in the highly sort after rare disease market. -
News Pharma Supply Chain People Moves
The latest appointments and promotions across the pharmaceutical supply chain. -
News Merck to donate new Ebola vaccine to defend against outbreaks in Uganda
Pharmaceutical giant Merck has announced they will be speeding up the processing of a new vaccine against the latest strain of the Ebola virus, to be donated to a global non-profit organisation for distribution -
News CPHI Podcast Series: Driving innovation with pharmaceutical startups
The latest episode in the CPHI Podcast Series explores how startups are driving innovation by taking high-risk approaches and doing business with greater agility. -
News Greener and efficient processes: Quaternary Ammonium Salts
Quaternary Ammonium Salts play a crucial part in Organic Chemistry processes at many major industries. Discover why.
-
News Biosimilars save patients $11B annually, but barriers to adoption remain in US market
Biosimilars introduce competition into the biologics market, driving down prices and increasing patient access. -
News WHO recommends use of two monoclonal antibody treatments against Ebola
The health body recommended use of treatments by Regeneron and Ridgeback Bio
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance




.png)


